Abstract
Application of nickel in different industries has been developed and so contamination of natural water is a great concern due to its potentially toxic effects on living beings. Therefore, fast monitoring of Ni2+ in aqueous samples is important. In this work, we fabricated a sensitive optical sensor for determination of nickel in mineral water samples and hydrogen peroxide solutions. The optode was prepared by incorporation of 1-(2-pyridylazo)-2-naphthol and sodium tetraphenylborate in a plasticized poly (vinyl chloride) membranes containing dioctyladipate as a plasticizer. The influence of several parameters such as pH, base matrix, solvent mediator and ligand concentration were optimized. Comparison the obtained results with previously reported sensors revealed that the proposed method, in addition to fast and simplicity, provided good linear range (1.70–85.20 µmol L-1) and low detection limit (0.17 µmol L-1). The precision (relative standard deviation) was better than 1.55% for 7 replicate determinations of 17.10 µmol L-1 of Ni in various membranes.
Author Contributions
Academic Editor: Dr.Praveen Kumar Sharma, Phagwara
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2016 Saeed Babaee, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Nowadays, the pollution of natural water by heavy metals is a great concern due to their potentially toxic effects on living beings, therefore the detection and monitoring of toxic metals in water samples is necessary and very important 1.
Due to wide applications of nickel in different industries 2, 3, 4, 5, 6, its contamination of natural water leads to serious environmental hazards. Nickel and some its compounds can cause an allergic reaction, asthma, lung cancer and leukemia in human body originates 7, 8, 9, 10. Amount of nickel should not be more than 1.70 µmol L-1 in drinking water resources 11.
Hydrogen peroxide is used as a disinfectant, oxidizer and so forth in usual applications 12. High concentration of hydrogen peroxide can be used either as a monopropellant or as an oxidizer for military applications 13. If cationic impurities exist in commercial grade of hydrogen peroxide solutions, its purification to military grade (over 85%) can cause to explosion. So nickel concentration in commercial H2O2 solutions must be lower than 0.68 µmol L-114. Because of oxidation medium or presence of oxygen bubbles in H2O2 solutions, it is difficult to direct determining any ions before initial pretreatments. Therefore, it is important to develop a safe, selective and sensitive technique for the rapid measurement of Ni2+ in different media.
Determination of nickel is performed by several techniques such as x-ray absorption spectroscopy 15, flame and electro thermal atomic absorption spectrometry 16, 17, 18, 19, 20, atomic emission spectrometry 21, spectrophotometry 22, 23 and fluorescence spectroscopy 24. Among the methods, spectrophotometric methods offer many appealing characteristics including: simple instrumentation, rapid response times and easy operation. These properties are desirable to the future design and development of portables analytical devices for nickel analysis.
Recently, an interest has been increased on the development of optical sensors compared to electrochemical sensors 25. These sensors have better analytical characteristics26 and they do not require internal and external reference devices, their time preconditioning are short and are not subjected to electrical noise 27. The choice of the optode matrix is governed by the parameters such as permeability for analyte, cost, good mechanical properties and immobilization suitability for chromophore along with uptaking 28, 29, 30.
The most widely used polymers in optical sensors are poly (vinyl chloride) groups. They have many desirable features and compare well with sol–gel matrices for most applications 31. Several optodes have been reported in trace analysis of different analytes such as metal ions, anions and organic compounds 32, 33, 34, 35, 36, 37.
1-(2-pyridylazo)-2-naphthol) PAN) is a red solid which is readily soluble in common organic solvents such as methanol, ethanol etc. It forms coloured complexes with a large number of metal ions 38.
In this research, we introduced a selective, sensitive and rapid method based on PVC sensor for spectrophotometric determination of nickel by using PAN in water and hydrogen peroxide samples. According to the best of our knowledge, although analysis of Ni2+ was reported by this method previously but there is no information for monitoring of this cation in H2O2 media.
Experimental
Chemicals
All the chemicals were of analytical grade. 1-(2-pyridylazo)-2-naphthol )PAN), Low molecular weight poly vinyl chloride (LPVC, mol wt ~ 48000 g mol-1), dioctyladipate (DOA) and sodium tetraphenylborate (NaTPB) were used without purification. Nickel solutions were prepared from a 17.04 mmol L-1 standard solution. A buffer of pH 6.0 was prepared from sodium hydroxide (0.046 M) and potassium hydrogenphthalate (0.05 M) solutions 39.
Apparatus
Absorption measurements were carried out on a Hitachi-U 3310 model Lambada-25 double beam UV-Vis spectrophotometer. The pH of the solutions was measured by a Metrohm model 691 pH/Ion Meter using a combined glass electrode.
Membrane Preparation
The membrane consisted appropriate amounts of active components. 30.0 mg of PVC, 75.0 mg of DOA, 8.0 mg of PAN and 5.0 mg of NaTPB were transferred in a glass vial and dissolved into 1 ml THF. The solution was immediately shaken vigorously to achieve complete homogeneity. A glass plate (1×9×50 mm3) was cleaned with pure THF and then placed in the spin-on device. Ninety micro liters of the above solution was injected to the glass plate. After 30 s spinning, at rotation frequency of 600 rpm, the membrane was located in ambient air and allowed to dry in air for few minutes.
Procedure
The sensing membrane (optode) was placed in a beaker filled with 20 ml of the test solutions containing EDTA (1.0×10-2 mol L-1) and different concentration of Ni2+ (1.70 - 85.20 µmol L-1) at pH 6.0. After 10 min the optode was mounted into the spectrophotometer directly and its net absorbance was recorded at 570 nm against a blank membrane.
Water samples (Mineral and river) were collected in 1 L amber glass bottles from Haraz re(Anahita, Polur) and Jajrud (Lavasan) rivers in Iran. After sample pretreatments 34, 3 ml of each sample was spiked with appropriate amount of Ni (II) and was subjected to the above procedure.
Hydrogen peroxide (10-20%) samples were prepared freshly from commercial hydrogen peroxide (30%) solution after filtration. Then, each of samples after Ni (II) spiking was subjected to the membrane methodology.
Results and Discussion
Preliminary Investigations
1-(2-pyridylazo)-2-naphthol )PAN) was recommended as a spectrophotometric reagent by Cheng and Bray where it gives a red dish colored chelates with metal ions as an example of Ni (II) 40. Because PAN is organic, it is efficiently immobilized in the hydrophobic part of the membrane without prior lyophilization 41. It was observed that by optimized fabricating a sensor from incorporation of PAN in a plasticized PVC membrane containing DOA, the determination of nickel could be practicable spectrophotometrically.
When nickel ions diffused into the membrane, they formed a complex with PAN so the membrane colors changed from yellow to red. The absorption spectra of the PAN optodes in different concentrations of Ni (λmax=570 nm) are shown in Figure 1
Figure 1.Absorption spectra of the proposed optode at various concentrations of Ni2+: (1) 1.70, (2) 3.41, (3) 8.52, (4) 13.64, (5) 17.04, (6) 25.56, (7) 51.12, (8) 85.20 µmol L-1; Conditions: pH=6.0; T=25◦C; response time=10 min; membrane layer containing 25.42% PVC, 63.56% DOA, 6.78% PAN and 4.24% NaTPB.
Membrane Composition
The response characteristics and working concentration range of each optical sensor depends significantly on the different ingredients such as base matrix, solvent mediator, ionophore and additive used in the membrane structure. Therefore the sensor matrix should be selected, firstly. In comparison of different polymers, low molecular weight PVC was observed as the best selection for the membrane base. This choice was due to several parameters such as appropriate transmittance, suitable immobilization of PAN without any leakage, good mechanical stability and reliable permeability to Ni2+ ions.
The nature of plasticizer is important and must be physically adaptable with polymer. In order to have a homogenous organic phase, several plasticizers such as dibutylphthalate (DBP), tributylphosphate (TBP), dioctylphthalate (DOP), ortho-nitrophenyloctyl ether (O-NPOE) and DOA were tested as potential plasticizers. From the graphs in Figure 2 (a and b), it was concluded that the membranes containing DBP, TBP, DOP and O-NPOE did not produced a suitable signals because of either improper physical properties with LPVC or leakage of PAN from the membrane. We found that DOA was the best selection with respect to good physical properties, the highest sensitivity and the lowest leakage.
Figure 2.Effect of plasticizer nature on the response of the membrane after 10 min (a) and on the membrane leakage% after 30 min (b). Conditions: 34.08 µmol L-1 Ni2+; T=25◦C; membrane layer containing 30.0 mg of PVC, 75.0 mg of each plasticizers, 8.0 mg PAN.
As shown in Table 1
(membrane no. 1-5), the sensors with a weight ratio of DOA to LPVC as 2.5 provided best absorbance. Thus, 30 mg of PVC and 75 mg of DOA were selected as the optimum values. A decrease of the Ni2+ uptaking efficiency, at values lower than 75 mg, is explained by improper solidity of the optode that led to low diffusion of analyte cations into the membrane. At quantities more than 75 mg, flexibility of the optode increased, led to ionophore leakage into the test solution.
Effect of different amounts of PAN on the membrane response is observed in Table 1. As seen in membrane no. 6-10, the absorbance increased by increasing amounts of PAN up to 8 mg and decreased at higher amounts that were resulted from membrane leakages. Therefore, 8 mg PAN was selected as optimum value.
Due to the complete mass transfer of Ni2+ ions into the membrane and decreasing of response time, the presence of an anionic additive such as NaTPB facilitates the ion-exchange equilibrium 42. The effect of NaTPB was investigated in the range 3.0-7.0 mg (membrane no. 11-15 of Table 1). It is shown that the highest absorbance is recorded by using 5.0 mg of NaTPB.
Table 1. Effects of membrane composition on the absorbance of the proposed optode| Membrane | LPVC (mg) | DOA (mg) | PAN (mg) | NaTPB (mg) | Response time (min) | Absorbance (570 nm)a |
| 1 | 30 | 65 | 8 | 5 | 10 | 0.197 ± 0.021 |
| 2 | 30 | 70 | 8 | 5 | 10 | 0.285 ± 0.012 |
| 3 | 30 | 75 | 8 | 5 | 10 | 0.357 ± 0.005 |
| 4 | 30 | 80 | 8 | 5 | 10 | 0.315 ± 0.009 |
| 5 | 30 | 85 | 8 | 5 | 10 | 0.177 ± 0.018 |
| 6 | 30 | 75 | 4 | 5 | 10 | 0.214 ± 0.017 |
| 7 | 30 | 75 | 6 | 5 | 10 | 0.303 ± 0.011 |
| 8 | 30 | 75 | 8 | 5 | 10 | 0.357 ± 0.005 |
| 9 | 30 | 75 | 10 | 5 | 10 | 0.348 ± 0.008 |
| 10 | 30 | 75 | 12 | 5 | 10 | 0.325 ± 0.010 |
| 11 | 30 | 75 | 8 | 3 | 10 | 0.231 ± 0.024 |
| 12 | 30 | 75 | 8 | 4 | 10 | 0.314 ± 0.011 |
| 13 | 30 | 75 | 8 | 5 | 10 | 0.358 ± 0.006 |
| 14 | 30 | 75 | 8 | 6 | 10 | 0.326 ± 0.017 |
| 15 | 30 | 75 | 8 | 7 | 10 | 0.280 ± 0.032 |
| 16 | 30 | 75 | 8 | 5 | 2 | 0.132 ± 0.023 |
| 17 | 30 | 75 | 8 | 5 | 6 | 0.300 ± 0.011 |
| 18 | 30 | 75 | 8 | 5 | 10 | 0.358 ± 0.004 |
| 19 | 30 | 75 | 8 | 5 | 12 | 0.359 ± 0.006 |
| 20 | 30 | 75 | 8 | 5 | 14 | 0.358 ± 0.005 |
Effects of pH
The influence of media pH on the sensor response was studied in the range 4-8. As it is shown in Figure 3 optode absorbance increased at pH 6.0 and then decreased. At pH<6, protonation of the ligand prevents its reaction with Ni2+ ions and at pH>6.0, the response decreasing could be due to the hydrolysis of Ni2+ ions that is caused to incomplete diffusion of Ni2+ cations into the membrane. Therefore, a buffer with pH 6.0 was chosen in all experiments. In comparison, the optimum pH for Ni-PAN complex in aqueous solution has been reported as 6.5 43.
Figure 3.Effects of pH on the absorbance of the proposed optode; conditions: 34.08 µmol L-1 Ni2+; T=25◦C; response time=10 min; membrane layer containing 25.42% PVC, 63.56% DOA, 6.78% PAN and 4.24% NaTPB.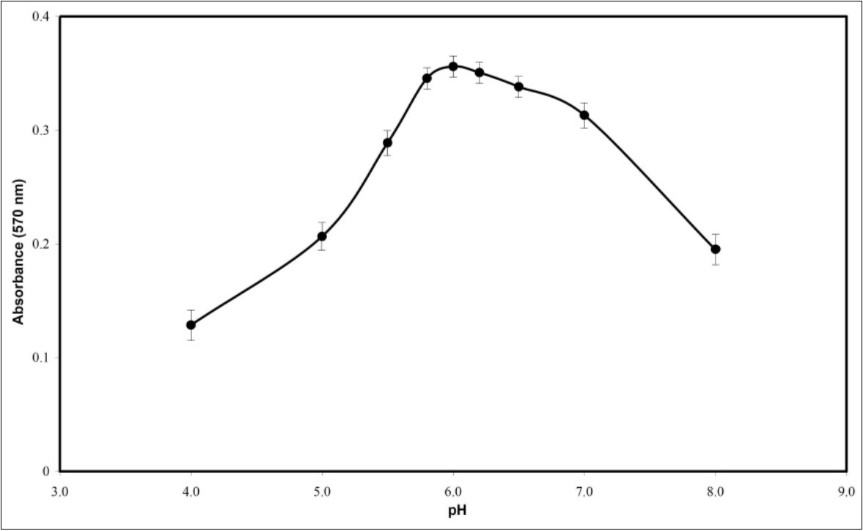
Response Time of Optode
Response time of optodes is defined as the diffusion time of the metal ions from solution into the membrane (slowest step in complexation process) 44. The effect of this parameter on the optode response was studied (Table 1 membrane no. 16-20). As seen, at least a time interval of 10 min is required for quantitative uptake at room temperature. It was observed that the optode response remained constant for more than 2 hours.
Membrane Properties
The properties of the optode membrane were measured by recording absorbance changed at 570 nm from individual solutions of 8.52, 17.04 and 51.12 µmol L-1 of Ni. As it is seen in Figure 4 all of the three cases, the optodes reached to 98% absorbances after 10 minutes.
The stability of membranes was tested for 2 hours and during this period a mean difference of absorbances for the mentioned solutions was ±0.007. Also the membrane responses were stable for one month in air.
Figure 4.Response time of the optode membranes for different concentrations of nickel, Conditions: pH=6.0; T=25◦C; membrane layer containing 25.42% PVC, 63.56% DOA, 6.78% PAN and 4.24% NaTPB
The salting-out phenomenon on the optode response was investigated by adding different amounts of sodium nitrate. The results indicated that this parameter had no effect on the membrane response, up to 0.04 mol L-1 of NaNO3 and above this concentration it was reduced slightly. This is due to a decrease in the activity of Ni2+ ions at higher concentration of electrolyte which reduces the interaction of nickel (II) cation with PAN in the membrane.
The sensor regeneration was studied by using of different compounds such as hydrochloric acid, nitric acid, sulfuric acid, sodium fluoride and oxalic acid in different concentrations. It was found that all of the reagents could not regenerate the optode membrane thoroughly and thus the membrane could be used as a probe (single test).
Analytical Characteristics
Table 2 summarizes the analytical characteristics of the optimized membrane. In this manner dynamic linear range was resulted within 1.70–85.20 µmol L-1 of nickel and detection limit was 0.17 µmol L-1. Also the relative standard deviation (RSD %) for 7 replicate determinations of 17.10 µmol L-1 of Ni+2 in various membranes was 1.55% and so the method is reproducible during the experiments.
Table 2. Analytical characteristics of the proposed sensor.| Regression equation (n=12) | A = 0.1721C + 0.018, r = 0.9991 |
| Linear range (µmol L-1) | 1.70-85.20 |
| Limit of detection (µmol L-1)a | 0.17 |
| Reproducibility (RSD%)b | 1.55 |
Table 3 presents a comparison between the proposed optode and the other sensors for determination of nickel previously 43, 45, 46, 47, 48. It is obvious that the obtained results of this work are comparable with these existing sensors. In some cases, it provides better linearity range and detection limit.
Table 3. Comparison of optical sensor papers for Ni (II) determination| Membrane type | Ionophore | pH | LDRa (M) | LODb (M) | Ref. |
| Nafion | 1-(2-pyridylazo)-2- naphthol | 6.5 | 2×10-5-12×10-5 | 1.7×10-5 | 43 |
| ″ | 2-(5-bromo-2-pyridylazo)-5-(diethylamino) phenol | 6.5 | 5. 1×10-6-3.4×10-2 | 8.5×10-6 | 45 |
| PVC | 2-amino-1-cyclopentene-1-dithiocarboxylic acid | 4-6.5 | 5.0×10-6 -1.0×10-3 | 5.2×10-7 | 46 |
| ″ | 1,2-di(o-salicylaldiminophenylthio)ethane | 6 | 1.0 × 10−5-5.0 × 10−3 | 8.5×10−6 | 47 |
| Triacetyl cellulose-optical | triazene-1-oxide derivative | 5.7 | 1.18×10−9-7.34×10−5 | 1.0×10−9 | 48 |
| PVC | 1-(2-pyridylazo)-2- naphthol | 6 | 1.70×10-6-8.52×10-5 | 1.70×10-7 | This work |
Study of Interferences
The selectivity of this sensor for determination of 8.52 µmol L-1 of Ni+2 was summarized in Table 4. The tolerance limit was defined as the concentration of added ion causing less than ±5 ٪relative error.
From Table 4, presence of alkaline metals and anions such as sulfate, chloride and others did not have adverse effects on nickel uptake. As seen, bivalent and some trivalent cations can interfere at different ratios. For elimination of these interferences, EDTA was selected as a masking agent. The effect of EDTA interfering was investigated firstly. It was resulted that EDTA2- did not affect (causing less than 4% negative deviation) on Ni measurement up to 1.0×10-2 mol L-1. Thus, the presence of EDTA in the test solution can effectively mask 0.2 mmol of any interfering cations. Hence, the proposed membrane is a selective sensor for nickel monitoring in water and H2O2 samples.
Table 4. Tolerance limits of diverse ions on the Ni+2 (8.52 µmol L-1) determination| Foreign ions | Tolerance Ratio (M:[Ni+2]) |
| Cations | |
| Na+, K+, Li+ and NH4+ | 83.33403 |
| Ca2+, Mg2+, Ba2+, Sr2+, Cr3+, UO22+ and ZrO22+ | 4.167361 |
| Mn2+, Fe2+, Fe3+, Al3+, Zn2+, Ga3+ and Cu2+ | 2.084028 |
| Co2+, Cd2+ and Pb2+ | 0.417361 |
| Anions | |
| SO42-, CO32-, NO3-, NO2-, BO3- and HCO3- | 83.33403 |
| I-, HPO42- and PO43- | 41.66736 |
| CN-, Cl- and F- | 0.834028 |
Method Application
The results of applicability of the membrane methodology are presented in Table 5. As shown, the mean recoveries for the addition of different concentrations of nickel to the water samples and H2O2 solutions were in the range of 98-104% and 98-105% respectively. Therefore, the proposed sensor can be successfully applied for the determination of nickel in the mentioned samples.
Table 5. Determination of Ni2+ in different spiked sample| Samplesa | Ni2+ (µmol L-1) | ||
| Added | Foundb | Recovery (%) | |
| Mineral | - | n.d.c | - |
| 1.7 | 1.77 ± 0.34 | 104 | |
| 13.63 | 13.46 ± 0.17 | 99 | |
| River | - | n.d. c | - |
| 6.82 | 6.64 ± 0.34 | 98 | |
| 17.04 | 17.38 ± 0.17 | 102 | |
| H2O2 (10%) | - | 1.87 ± 0.51 | - |
| 5.11 | 7.16 ± 0.34 | 103 | |
| 11.93 | 13.63 ± 0.17 | 99 | |
| H2O2 (20%) | - | 2.90 ± 0.51 | - |
| 3.41 | 6.47 ± 0.34 | 105 | |
| 13.63 | 16.19 ± 0.34 | 98 | |
Conclusion
The proposed optode is a precise, low cost and sensitive device for determination of nickel, based on PVC membrane. Also the proposed method, in addition to fast and simple, provides a wide dynamic range, reliable reproducibility and a good limit of detection. EDTA was used as masking agent and the method could be made selective in this way. A comparison of the proposed optode with the previously reported sensors indicates that the proposed method in some cases provides wider linear range and lower detection limit. Finally, the fabricated sensor can be successfully applied to nickel monitoring in water and hydrogen peroxide samples. According to the best of our knowledge no manufactured optode has been reported in the literature for the determination of Ni in H2O2 solutions.
References
- 1.I M Steinberg, Lobnik A, O S Wolfbeis. (2003) Characterisation of an optical sensor membrane based on the metal ion indicator Pyrocatechol Violet. , Sens. Actuators 90, 230-235.
- 2.Repo E, J K Warchol, T A Kurniawan, Sillanpaa M E T. (2010) Adsorption of Co(II) and Ni(II) by EDTA- and/or DTPA-modified chitosan: Kinetic and equilibrium modeling. , Chem. Eng. J 161, 73-82.
- 3.Fu F, Chen R, Xiong Y. (2007) Comparative investigation of N, N-bis(dithiocarboxy) piperazine and diethyldithiocarbamate as precipitants for Ni(II) in simulated wastewater. , J. Hazard. Mater 142, 437-442.
- 4.Demirbas E, Kobya M, Oncel S, Sencan S. (2002) Removal of Ni (II) from aqueous solution by adsorption onto hazelnut shell activated carbon: equilibrium studies. , Bioresour. Technol 84, 291-293.
- 5.Kadirvelu K, Senthilkumar P, Thamaraiselvi K, Subburam V. (2002) Removal of Ni (II) from aqueous solution by adsorption onto hazelnut shell activated carbon: equilibrium studies. , Bioresour. Technol 81, 87-90.
- 6.Cempel M, Nikel G. (2006) Nickel: A Review of Its Sources and Environmental Toxicology. , Pol. J. Environ. Stud 15, 375-382.
- 7.Costa M, T L Davidson, Chen H, Ke Q, Zhang P et al. (2005) Nickel carcinogenesis: epigenetics and hypoxia signaling. , Mutat. Res 592, 79-88.
- 8.R S Amais, J S Ribeiro, M G Segatelli, Yoshida I V P, P O Luccas. (2007) Assessment of nanocomposite alumina supported on multi-wall carbon nanotubes as sorbent for on-line nickel preconcentration in water samples . , Sep. Purif. Technol 58, 122-128.
- 9.Dadfarnia S, Shabani A M H, Shirani Bidabadi M, Jafari A A. (2010) A novel ionic liquid/micro-volume back extraction procedure combined with flame atomic absorption spectrometry for determination of trace nickel in samples of nutritional interest. , J. Hazard. Mater 173, 534-538.
- 10.Ahmadpour A, Tahmasbi M, Rohani Bastami T, Amel Besharati J. (2009) Rapid removal of cobalt ion from aqueous solutions by almond green hull.J.Hazard.Mater. 166, 925-930.
- 11.United State Departmentof health and human services, Public Health Service Agency for Toxic Substances and Disease Registry, Agency for toxic substances and disease registry (ATSDR). http://www.atsdr.cdc.gov/toxfaq. html
- 13.J E Love, W H Stillwell. (1959) The Hydrogen-peroxide rocket reaction-control system for the X-1B research airplane. , Washington
- 14.MIL-PRF-16005F. (2003) Performance specification-Propellant-Hydrogen peroxide, Defense Standardization Program office (DLSC-LM). , Virginia
- 15.Xu Y, Axe L, Boonfueng T, Tyson T A, Trivedi P. (2007) Ni(II) complexation to amorphous hydrous ferric oxide: An X-ray absorption spectroscopy study. , J. Colloid Interface Sci 314, 10-17.
- 16.Baytak S, A R Turker. (2006) Determination of lead and nickel in environmental samples by flame atomic absorption spectrometry after column solid-phase extraction on Ambersorb-572 with EDTA. , J. Hazard. Mater 129, 130-136.
- 17.Ciftci H, Yalcin H, Eren E, Olcucu A, Sekerci M. (2010) Enrichment and determination of Ni2+ ions in water samples with a diamino-4-(4-nitro-phenylazo)-1H-pyrazole (PDANP) by using FAAS. , Desalination 256, 48-53.
- 18.Soylak M, Kars A, Narin I. (2008) Coprecipitation of Ni2+, Cd2+ and Pb2+ for preconcentration in environmental samples prior to flame atomic absorption spectrometric determinations. , J. Hazard. Mater 159, 435-439.
- 19.Ghaedi M, Tavallali H, Shokrollahi A, Zahedi M, Montazerozohori M. (2009) Flame atomic absorption spectrometric determination of zinc, nickel, iron and lead in different matrixes after solid phase extraction on sodium dodecyl sulfate (SDS)-coated alumina as their bis (2-hydroxyacetophenone)-1, 3-propanediimine chelate. , J. Hazard. Mater 166, 1441-1448.
- 20.Campos R C D, Santos H R D, Grinberg P. (2002) Determination of copper, iron, lead and nickel in gasoline by electrothermal atomic absorption spectrometry using threecomponent solutions. , Spectrochim. Acta. B 57, 15-28.
- 21.Koizumi C, Usuda K, Hayashi S, Dote T, Kono K. (2005) Evaluation of Urinary Nickel using Inductively Coupled Plasma Argon Emission Spectrometry. , Bull. Osaka Med. Coll 51, 1-7.
- 22.L S Sarma, J R Kumar, K J Reddy, Thriveni T, A V Reddy. (2008) Development of highly sensitive extractive spectrophotometric determination of nickel(II) in medicinal leaves, soil, industrial effluents and standard alloy samples using pyridoxal-4-phenyl-3-thiosemicarbazone. , J. Trace Elem. Med. Biol 22, 285-295.
- 23.Ghaedi M. (2007) Selective and sensitized spectrophotometric determination of trace amounts of Ni(II) ion using -benzyl dioxime in surfactant media. , Spectrochim. Acta. A 66, 295-301.
- 24.Ressalan S, Iyer C S P. (2005) Absorption and fluorescence spectroscopy of 3-hydroxy-3-phenyl-1-o- carboxyphenyltriazene and its copper (II), nickel (II) and zinc (II) complexes: a novel fluorescence sensor. , J. Luminescence 111, 121-129.
- 25.M R Ganjali, Hosseini M, Hariri M, Faridbod F, Norouzi P. (2009) Novel erbium (III)-selective fluorimetric bulk optode. , Sens. Actuators. B 142, 90-96.
- 26.W R Seitz. (1991) Optical Ion Sensing Fiber Optic, in Chemical Sensors Biosensors II (O.S. Wolfbeis Ed.),CRC.Press,Bocaraton.
- 27.Absalan G, Asadi M, Kamran S, Torabi S, Sheikhian L. (2010) Design of a cyanide ion optode based on immobilization of a new Co (III) Schiff base complex on triacetylcellulose membrane using room temperature ionic liquids as modifiers. , Sens. Actuators. B 147, 31-36.
- 28.Kalyan Y, A K Pandey, P R Bhagat, Acharya R, Natarajan V. (2009) Membrane optode for mercury(II) determination in aqueous samples. , J. Hazard. Mater 166, 377-382.
- 29.M R Ganjali, Zare Dorabei R, Norouzi P. (2009) Design and construction of a novel optical sensor for determination of trace amounts of dysprosium ion. , Sens. Actuators. B 143, 233-238.
- 30.Kalyan Y, A K Pandey, Naidu G R K, Reddy A V R. (2009) Membrane optode for uranium(VI) ions preconcentration and quantification based on a synergistic combination of 4-(2-thiazolylazo)-resorcinol with 8-hydroxyquinaldine. , Spectrochim. Acta. A 74, 1235-1241.
- 31.Rastegarzadeh S, Pourreza N, Saeedi I. (2010) An optical chemical sensor for thorium (IV) determination based on thorin. , J. Hazard. Mater 173, 110-114.
- 32.Safavi A, Bagheri M. (2004) Design and characteristics of a mercury (II) optode based on immobilization of dithizone on a triacetylcellulose membrane. , Sens. Actuators. B 99, 608-612.
- 33.Khayatzadeh Mahani M, Divsar F, Chaloosi M, Ghanadi Maragheh M, A R Khanchi. (2008) Simultaneous determination of thorium and uranyl ions by optode spectra and chemometric techniques. , Sens. Actuators. B 133, 632-637.
- 34.Beiraghi A, Babaee S, Roshdi M. (2011) Spectrophotometric Determination of Trace Amounts of Beryllium Using 1,8-Dihydroxyanthrone as a New Chromogenic Reagent. , J. Hazard. Mater 190, 962-968.
- 35.Ensafi A A, Katiraeifar A, Meghdadi S. (2009) Highly selective optical-sensing film for lead (II) determination in water samples. , J. Hazard. Mater 172, 1069-1075.
- 36.Shamsipur M, Sadeghi M, Alizadeh K, Bencini A, Valtancoli B. (2010) Novel fluorimetric bulk optode membrane based on 5,8-bis ((5-chloro-8-hydroxy-7-quinolinyl)methyl)-2,11-dithia-5,8-diaza-2,6-pyridinophane for selective detection of lead(II) ions.Talanta. 80, 2023-2033.
- 37.Choi M M F, Chung K O P, Wu X. (2002) Nicotine derivative optode membrane with nonactin as Ionophore. , Talanta 56, 1027-1038.
- 38.Marczenko Z. (1986) Separation and Spectrophotometric Determination of Elements,John Wiley and Sons,NY.
- 39.Lurie J J. (1978) . , Handbook of Analytical Chemistry, English Translation,Mir Publishers,Moscow
- 40.K L Cheng, R H Bray. (1955) 1-(2-Pyridylazo)-2-naphthol as possible analytical reagent. , Anal. Chem 27, 782-785.
- 41.L D Coo, C J Belmonte. (2002) Nafion-based optical sensor for the determination of selenium in water samples. , Talanta 58, 1063-1069.
- 42.Shamsipur M, Poursaberi T, A R Karami, Hosseini M, Momeni A. (2004) A novel optical chemical sensor for thallium(III) determination using 4-(5-bromo-2-pyridylazo)-5-(diethylamino)-phenol. , Anal. Chim. Acta 501, 55-60.
- 43.Aksuner N, Henden E, Yilmaz I, Cukurovali A. (2012) A novel optical chemical sensor for the determination of nickel(II) based on fluorescence quenching of newly synthesized thiazolo-triazol derivative and application to real samples. , Sens. Actuators B 269, 166-167.
- 44.Fouladgar M, Ensafi A. (2010) A novel optical chemical sensor for thallium(III) determination using 4-(5-bromo-2-pyridylazo)-5-(diethylamino)-phenol. Sens. Actuators B. 143, 590-594.
- 45.J E Madden, T J Cardwell, R W Cattrall, L W Deady. (1996) Nafion-based optode for the detection of metal ions in flow Analysis. , Anal. Chim. Acta 319, 129-134.
- 46.M K Amini, Momeni Isfahani T, J H Khorasani, Pourhossein M. (2004) Development of an optical chemical sensor based on 2-(5-bromo-2 pyridylazo)-5-(diethylamino) phenol in Nafion for determination of nickel ion. , Talanta 63, 713-720.
Cited by (3)
- 1.Pimsin Nipaporn, Kongsanan Niradchada, Keawprom Chayanee, Sricharoen Phitchan, Nuengmatcha Prawit, et al, 2021, Ultratrace Detection of Nickel(II) Ions in Water Samples Using Dimethylglyoxime-Doped GQDs as the Induced Metal Complex Nanoparticles by a Resonance Light Scattering Sensor, ACS Omega, 6(23), 14796, 10.1021/acsomega.1c00190
- 2.Mao Xiaoxia, Liu Shaowei, Su Benyue, Wang Dejin, Huang Zhan, et al, 2020, Luminescent europium(III)-organic framework for visual and on-site detection of hydrogen peroxide via a tablet computer, Microchimica Acta, 187(7), 10.1007/s00604-020-04379-4
- 3.Alshehri Reem F., Amin Alaa S., Darwish Eman R., 2023, Ultrasensitive and highly selective detection of nickel ion by two novel optical sensors, Analytical and Bioanalytical Chemistry, 415(23), 5695, 10.1007/s00216-023-04845-x
