HIV-Associated Peripheral Neuropathy and Antiretroviral Therapy: A Prospective Study from a Tertiary Care Centre in South India
Abstract
Background
Peripheral neuropathy (PN) is a common and debilitating complication in people living with HIV (PLHIV). While HIV itself contributes to neuropathy, certain antiretroviral therapy (ART) drugs, particularly nucleoside reverse transcriptase inhibitors (NRTIs) such as stavudine (d4T) and zidovudine (AZT), are known for their neurotoxic effects.
Objectives
To evaluate the impact of ART on HIV-associated peripheral neuropathy (HIV-PN) and to determine whether certain ART regimens increase the risk or severity of neuropathy.
Materials and Methods
A cross-sectional study was conducted among 158 HIV-positive patients. Neuropathy was diagnosed using clinical criteria, Total Neuropathy Score (TNS), and nerve conduction studies (NCS). Patients were grouped based on their ART regimen, and statistical analysis was performed to assess the association between ART type and peripheral neuropathy severity.
Results
It was noted that patients on older NRTIs (stavudine, zidovudine) had significantly higher rates of peripheral neuropathy (p=0.002) and tenofovir-based regimens were associated with lower peripheral neuropathy prevalence (p=0.01). There was a significant correlation between the duration of ART exposure and peripheral neuropathy severity (p<0.001), suggesting a cumulative neurotoxic effect.
Conclusion
Older ART regimens, particularly stavudine and zidovudine, significantly contribute to HIV-PN. The study supports the WHO recommendation to phase out neurotoxic ART and highlights the importance of early ART regimen optimisation to prevent long-term neurological complications.
Author Contributions
Academic Editor: Shivaji Kashinath Jadhav, Orange Health Infectious Laboratory, Bangalore, India
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2025 Anagha Rao
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have no conflict of interest to declare.
Citation:
Introduction
Peripheral neuropathy (PN) is one of the most common neurological complications of HIV, affecting 30–67% of PLHIV. 1 It presents as distal sensory neuropathy (DSN), characterised by burning pain, paresthesia, and sensory loss in a stocking-glove distribution. 2
The pathogenesis of HIV-associated peripheral neuropathy (HIV-PN) is complex and involves direct HIV neurotoxicity (with viral proteins inducing neuroinflammation), immune activation (leading to chronic oxidative stress), and antiretroviral-induced mitochondrial toxicity (particularly with older NRTIs). 3
The rationale behind this study was to identify whether early ART was linked to high neuropathy rates due to stavudine and zidovudine. As with newer drugs (e.g., tenofovir, dolutegravir), peripheral neuropathy prevalence appears to be declining, although ART duration and cumulative toxicity may still play a role in peripheral neuropathy risk.
Objectives
· To assess the impact of ART regimens on HIV-PN prevalence and severity.
· To compare older neurotoxic NRTIs vs. newer ART formulations.
· To determine whether ART duration correlates with peripheral neuropathy severity.
Methodology
This was a cross-sectional observational study conducted at the out-patient/in-patient department in a tertiary care government-run hospital attached to Karnataka Medical College and Research Institute (KMCRI) in Hubballi, a city in the south Indian state of Karnataka, from January 2023 to December 2023. Patients presenting to the Medicine in-patient and Neurology out-patient departments were selected.
The study included HIV-positive individuals above 18 years of age, on cART for at least 6 months and presented to the clinic with symptoms suggestive of peripheral neuropathy (e.g., numbness, tingling, pain, or weakness). It excluded those patients with pre-existing diabetes mellitus or other known causes of neuropathy (e.g., chronic alcohol use, vitamin B12 deficiency), those with a history of neuromuscular disorders unrelated to HIV, patients with active opportunistic infections (e.g., TB, CMV neuropathy), and those on neurotoxic medications (e.g., isoniazid, metronidazole) unrelated to HIV treatment.
Demographic details (age, sex, body mass index, duration of HIV infection, and cART history) were collected from all the participants and neuropathic symptoms were assessed along with a thorough neurological assessment for sensory testing – vibration sensation (128 Hz at great toe and ankle joint), temperature sensation (hot and cold testing), pain sensation (pinprick), and deep tendon reflexes (ankle, knee, wrist, and elbow joints). The patients were categorised based on their ART regimen into one of four groups: stavudine-based (d4T), zidovudine-based (AZT), tenofovir-based (TDF), and dolutegravir-based (DTG) regimens.
Electrophysiological testing was performed in the form of nerve conduction studies (NCS) which measure the electrical conduction of peripheral nerves in the upper limb (median and ulnar nerves) and lower limb (Common peroneal, tibial, and sural nerves) by measuring the parameters – Motor nerve conduction velocity (NCV), Sensory NCV, Compound motor action potential (CMAP), sensory nerve action potential (SNAP), distal latency, and F-wave latency. This helps differentiate axonal degeneration from demyelination, providing an objective confirmation of the peripheral neuropathy and classifying its severity.
The Total Neuropathy Score (TNS) was used to quantify peripheral neuropathy (PN) severity on a scale of 0 to 44 – mild PN (<10), moderate PN (10-20), and severe PN (>20). Nerve conduction studies (NCS) were performed in a subset of symptomatic patients (n=115), and CD4+ counts were measured using flow cytometry within 3 months of enrollment.
The data was coded and recorded in an MS Excel spreadsheet program, and Windows SPSS v23 was used for data analysis. The Pearson correlation coefficient was used to assess the relationship between CD4+ and TNS scores. One-way ANOVA and logistic regression for subgroup comparisons. A p-value <0.05 was considered statistically significant. The study was approved by the Institutional Ethics Committee, and informed consent was obtained from all participants.
Results
Of the total study population (n=158), 30 were exposed to zidovudine (AZT), 59 were exposed to stavudine (D4T), 69 were exposed to tenofovir (TDF), 48 were to dolutegravir (DTG), 56 to efavirenz (EFV), and 54 to nevirapine (NVP) in their lifetime.
Among the study population (n=158), 37.4% (n=59) were exposed to Stavudine (D4T) containing regimen, 43.7% (n=69) were exposed to Tenofovir (TDF) containing regimen, and 19% (n=30) were exposed to Zidovudine (AZT) containing regimen. (Figure 1)
Figure 1.Initial regimens of HAART started among all study participants (n=158) (D4T – Stavudine, 3TC – Lamivudine, NVP – Nevirapine, EFV – Efavirenz, AZT – Zidovudine, DTG – Dolutegravir, TDF – Tenofovir .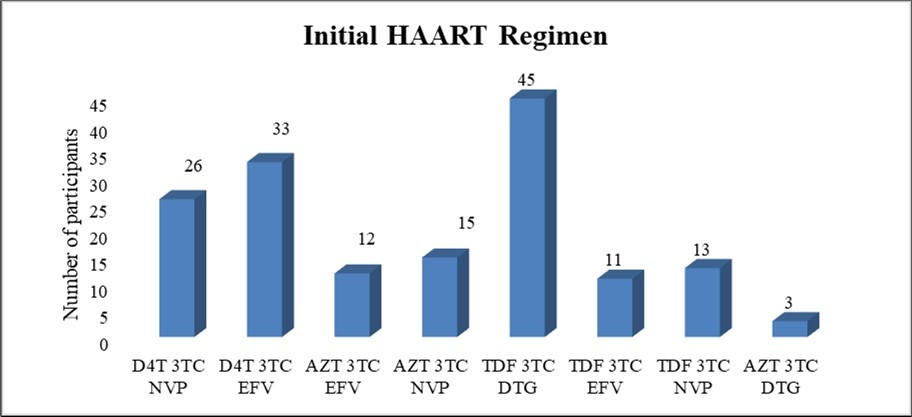
A significant correlation was noted between those individuals with exposure to stavudine (p<0.005), tenofovir (p=0.001), dolutegravir (p=0.015) and nevirapine (p=0.031) medications and the severity of peripheral neuropathy by the TNS in the present study. (Table 1)
Table 1. Results of the Student’s t-test for correlation between each of the HAART drugs and the peripheral neuropathy severity by the TNS among the study participants (p<0.05)| TN SCORE | t-test | ||||||
| HAART | Exposure | n | Mean | SD | Mean Difference | t | p value |
|---|---|---|---|---|---|---|---|
| Zidovudine | No | 128 | 14.18 | 11.25 | 1.68 | 0.723 | 0.471 |
| Yes | 30 | 12.5 | 12.3 | ||||
| Stavudine | No | 99 | 11 | 10.05 | 7.66 | 4.293 | <0.005 (Sig.) |
| Yes | 59 | 18.66 | 12.08 | ||||
| Tenofovir | No | 89 | 16.58 | 12.43 | 6.24 | 3.52 | 0.001 (Sig.) |
| Yes | 69 | 10.35 | 8.93 | ||||
| Dolutegravir | No | 110 | 15.31 | 11.92 | 4.76 | 2.448 | 0.015 (Sig.) |
| Yes | 48 | 10.54 | 9.56 | ||||
| Efavirenz | No | 102 | 13.74 | 10.95 | 0.35 | 0.186 | 0.853 |
| Yes | 56 | 14.09 | 12.37 | ||||
| Nevirapine | No | 104 | 12.45 | 11.25 | 4.12 | 2.174 | 0.031 (Sig.) |
| Yes | 54 | 16.57 | 11.41 | ||||
There was a significant correlation in the One-way ANOVA between the HAART initial regimen and the severity of the peripheral neuropathy by the TN score with p=0.001 (p<0.05) in the present study. (Table 2)
Table 2. Results of the One way ANOVA for correlation between the HAART initial regimen and the peripheral neuropathy severity by the TNS among the study participants (p<0.05) (D4T – Stavudine, NVP – Nevirapine, EFV – Efavirenz, AZT – Zidovudine, DTG – Dolutegravir, TDF – Tenofovir, 3TC – Lamivudine)| cART | TN SCORE | One Way ANOVA | ||||
| n | Mean | SD | Mean Difference | p value | ||
| HAART Initial regimen | D4T 3TC NVP | 26 | 19.77 | 10.53 | 3.784 | 0.001(Sig.) |
| D4T 3TC EFV | 33 | 17.79 | 13.27 | |||
| AZT 3TC EFV | 12 | 10.83 | 9.78 | |||
| AZT 3TC NVP | 15 | 16.00 | 14.02 | |||
| TDF 3TC DTG | 45 | 11.13 | 9.56 | |||
| TDF 3TC EFV | 11 | 6.55 | 7.26 | |||
| TDF 3TC NVP | 13 | 10.85 | 7.54 | |||
| AZT 3TC DTG | 3 | 1.67 | 2.89 | |||
Out of the total number of study participants (n=158), 70.9% (n=112) had a history of change in their cART regimen in the past. The common reasons for the change in regimen were toxicities in 57% (n=90), availability of a newer cART regimen as per NACO guidelines in 52.5% (n=83), co-infection/comorbidities in 40.5% (n=64), poor adherence to cART in 47.5% (n=75), and pregnancy in 4.4% (n=7) of the participants.
Among the toxicities reported as reasons for the change in cART regimen in study participants, GI toxicity was noted in 50% (n=79), lipodystrophy in 36.7% (n=58), anemia in 34.8% (n=55), nephrotoxicity in 26.6% (n=42), peripheral neuropathy in 23.4% (n=37) and rash in 3.2% (n=5).
Among the population sampled in our study (n=158), 89.9% (n=142) reported their current regimen of cART as TDF regimen while 7.6% (n=12) were on ALD regimen, 1.9% (n=3) on TLE regimen and 0.6% (n=1) on ZLN regimen. There was no significant correlation between the current regimen of cART and peripheral neuropathy by the TNS with a p=0.239 (p<0.05) in the present study.
Out of total number of participants (n=158), the total duration of HAART was >10y in 46.2% (n=73), 5-10y in 26.6% (n=42) of the participants, 1-5 years in 8.2% (n=13), 31 days-1 year in 3.2% (n=5), 6-30 days in 3.8% (n=6), and <5 days in 12% (n=19) of the study population.
There was a significant correlation in the one-way ANOVA, between the total duration of HAART and the peripheral neuropathy by the TNS scoring with a p=0.001 (p<0.05) in the present study.
The pairwise comparison (Tukey’s test) of respective groups of HAART duration for TNS severity showed a marginally significant correlation between those individuals with HAART duration of 6-30 days and >10y with p=0.046 (p<0.05) as well as a significant correlation between those with a HAART duration of 5-10y and >10y with a p= 0.008. (p<0.05)
Among the study population (n=158), 39.2% (n=62) had a history of defaulting their ART medications in the past or the present. Among the defaulters, 41.9% (n=26) defaulted their medications for 1-6 months, 25.8% (n=16) defaulted for 6m-1y duration, 19.4% (n=12) for a period of <1month, 11.3% (n=7) for 1-5y and 1 individual for >5 years duration.
The mean duration of defaulting HAART was 3.19 months in the present study. There was no significant correlation between the total duration of defaulting HAART and the peripheral neuropathy by the TNS scoring with p=0.103 (p<0.05).
The scatterplot diagram depicts a positive correlation between the total duration of defaulting HAART and TN score with a r² = 0.01698. (Figure 2)
Figure 2.Scatterplot diagram depicting the total duration of defaulting HAART vs TNS in the present study (r² = 0.01698)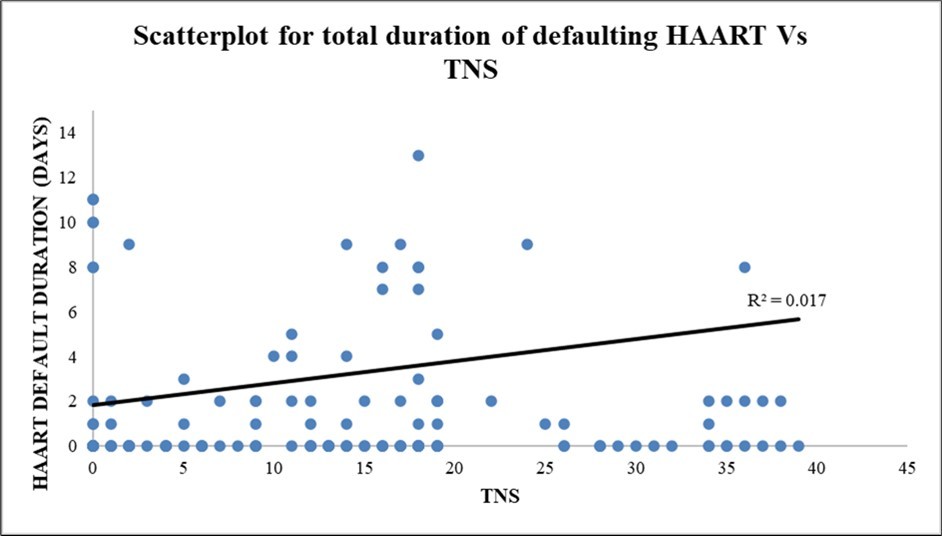
Using the TNS, the prevalence of neuropathic symptoms in our study was found to be 86% (136/158), out of which 5% had no symptoms but an abnormal TNS. Additionally, 8% with symptoms of peripheral neuropathy have a normal NCS. (Figure 3)
Figure 3.Mean TN scores in various groups in all study participants (n=158)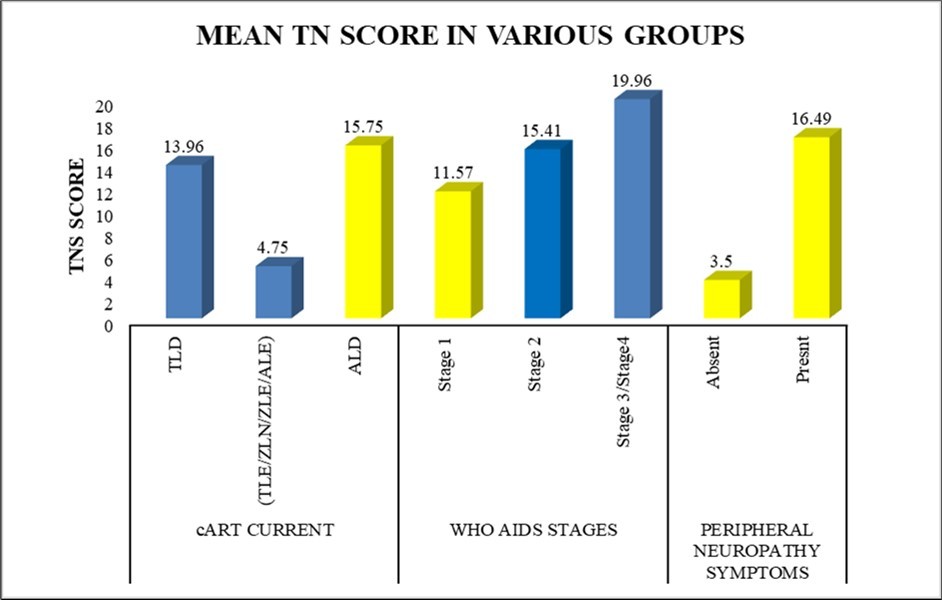
The electrophysiological findings (NCS subgroup, n=115) revealed 66 patients (57.4%) with axonal degeneration, 29 patients (25.2%) with demyelination, and the rest 20 patients (17.4%) with a mixed pattern.
Discussion
The present study showed that the use of older NRTIs (stavudine, zidovudine) is strongly associated with HIV-PN, while tenofovir and dolutegravir-based regimens have a lower risk of neuropathy. Notably, longer ART duration increases peripheral neuropathy risk, suggesting cumulative neurotoxicity.
There was a statistically significant association between the duration of HAART and peripheral neuropathy in our study, which is comparable to Schuldt et al 4, Tumusiime et al 5, Evans et al 6 but not to Gupta et al 7 and Opemo et al 8.
There was no significant association between the type of current HAART regimen and peripheral neuropathy in our study which is comparable to Schuldt et al 4, Opemo et al 8, and Shah et al 9 but not to Anastasi et al 10, who demonstrated a strong statistical significance.
There was a significant association between the duration of HIV seropositivity and peripheral neuropathy in our study, which is comparable to Schuldt et al 4, Tumusiime et al 5, Iyagba et al 11, and Anastasi et al 12. However, the study done by Amruth et al 13 did not reveal any significant correlation. The plausible reason is likely that till early 2017 in India, HAART was started at <350 cells/mm3, so HIV infected patients had to wait longer for HAART initiation.
Iyagba et al 11 also found that 13% (10-15%) of ART-naïve patients had developed peripheral neuropathy, suggesting that peripheral nerve injury occurs much earlier in the course of the disease, even with milder immunodeficient states. This consolidates the need to start HAART early, in HIV infected patients to prevent DSPN, an irreversible complication of HIV and thereby improve their quality of life. 11
There was a strong statistical correlation between the exposure to d-drugs (stavudine, zidovudine) and peripheral neuropathy in our study, which is comparable to Schuldt et al 4, Amruth et al 13, and Cherry et al 14 but not to Tumusiime et al 5 and Luma et al 15. Interestingly, the study by Evans et al 6 showed a significant association between protease inhibitor use and peripheral neuropathy.
In the study by Schuldt et al 4, peripheral neuropathy has an independent association with a history of d-drugs (aOR 1.88, 95% CI of 1.12–3.16). The excess risk for peripheral neuropathy associated with d-drug use remains after the exposure has stopped for years, suggesting non-reversible toxicity. 4
In the study by Amruth et al 13, those patients with DDI contained in the HAART regimen were four times more likely to develop HIV-PN (AOR = 4.33, 95% CI: 1.34, 14.00), more specifically due to prior exposure to Stavudine. 13
In the study done by Cettomai et al 16, when compared to those with no/mild neuropathy, participants with moderate/severe neuropathy by ACTG criteria were significantly older, had been diagnosed with HIV for a longer period, were more likely to be WHO stage 3 or 4, and were more likely to have ever used or to have discontinued stavudine due to peripheral neuropathy, similar to our study.
Our findings support WHO recommendations to phase out stavudine, and similar studies done in Africa and Asia also report higher neuropathy rates with zidovudine and stavudine.
Limitations
Due to the cross-sectional nature of our study, temporal relationships could not be established and causality cannot be inferred.
Conclusion
Our study demonstrates a significant association between older antiretroviral therapy (ART) regimens, particularly those containing stavudine and zidovudine, and the severity of HIV-associated peripheral neuropathy (HIV-PN).
Patients who were exposed to stavudine showed the highest prevalence of neuropathy (97.5%), followed closely by those on zidovudine (86.8%). In contrast, those on tenofovir-based or dolutegravir-based regimens showed significantly lower prevalence, highlighting the neuroprotective advantage of newer ART formulations. Furthermore, the duration of ART exposure was positively correlated with neuropathy severity, suggesting a cumulative neurotoxic effect over time.
These findings reinforce the World Health Organisation (WHO) recommendation to phase out neurotoxic d-drugs, especially stavudine. The data also emphasise the importance of early regimen optimization and routine neuropathy screening in long-term ART users. This is particularly crucial in low- and middle-income settings where stavudine may still be in use due to cost or accessibility.
Routine neurological monitoring, timely substitution of neurotoxic drugs, and increasing the availability of safer ART combinations must become an integral part of HIV care. Further longitudinal studies are necessary to evaluate the reversibility of neuropathy after ART modification and to explore the potential protective role of integrase inhibitors like dolutegravir in this context.
Key Recommendations
· Routine screening for HIV-PN in long-term ART users.
· Early substitution of neurotoxic NRTIs (e.g., stavudine, zidovudine) with safer alternatives.
· Educate clinicians on long-term ART-related neuropathy risks and encourage timely regimen review.
· Evaluate the role of integrase inhibitors in neuroprotection with future longitudinal studies.
References
- 1.Saylor D. (2018) Neurologic Complications of Human Immunodeficiency Virus Infection. Continuum (Minneap Minn). Oct;24(5, Neuroinfectious Disease): 1397-1421. doi: 10.1212/CON.0000000000000647. PMID: 30273245; PMCID: PMC8006925 .
- 2.Octaviana F, Yanuar Safri A, Imran D, Price P. (2019) HIV-Associated Sensory Neuropathy [Internet]. Demystifying Polyneuropathy - Recent Advances and New Directions. IntechOpen;. Available from: http://dx.doi.org/10.5772/intechopen.81176
- 3.Adem K S, Janakiraman B, Gebremeskel B F, Chala M B, Gelaw A Y et al. (2019) Epidemiology and factors associated with peripheral neuropathy among HIV infected patients in Gondar, Ethiopia: A cross-sectional study. PLoS One. 14(1), 30695060-6350981.
- 4.Schuldt A L, Bern H, Hart M, Gompels M, Winston A et al. (2023) Arenas-Pinto A; PIVOT Study Team. Peripheral Neuropathy in Virologically Suppressed People Living with HIV: Evidence from the PIVOT Trial. Viruses. 16(1), 38275937-10818628.
- 5.Tumusiime D K, Venter F, Musenge E, Stewart A. (2014) Prevalence of peripheral neuropathy and its associated demographic and health status characteristics, among people on antiretroviral therapy in Rwanda. BMC Public Health. 1306-10.
- 6.Evans S R, Ellis R J, Chen H, Yeh T M, Lee A J et al. (2011) Peripheral neuropathy in HIV: prevalence and risk factors. AIDS. 25(7), 919-28.
- 7.Pulin Kumar Gupta, Varun Vivek, Mahto Subodh, Hansraj, Anand Kuljeet et al. (2020) . Prevalence and Predictors of Distal Symmetric Polyneuropathy in Patients with HIV/AIDS not on Highly Active Anti Retroviral Therapy (HAART). The Journal of the Association of Physicians of India 68, 23-26.
- 8.Opemo D. (2020) Factors Associated with Occurrence of Peripheral Neuropathy among HIV/AIDS Clients at Kombewa Sub County Hospital of Kisumu County. , Kenya, Open Journal of Nursing 10, 665-675.
- 9.Shah H M, Patel A S. (2018) . CD4 Count and Opportunistic Infections in HIV Positive Patients with Neurological Manifestations. Natl J Community Med [Internet]. [cited 9(12), 893-6.
- 10.Anastasi J K, Devine D K, Capili B. (2021) Distal Sensory Peripheral Neuropathy: An Undervalued Determinant of Wellbeing. Health Educ Public Health. Oct;4(3): 450-454. doi: 10.31488/heph.169. Epub 28, 35224488-8870780.
- 11.Iyagba A, Onwuchekwa A. () HIV-associated peripheral neuropathies: a review. , Int J Med Res Rev 4(11), 2038-10.
- 12.Anastasi J K, Pakhomova A M. (2020) Assessment and Management of HIV Distal Sensory Peripheral Neuropathy: Understanding the Symptoms. J Nurse Pract. Apr;16(4): 276-280. doi: 10.1016/j.nurpra.2019.12.019. Epub 33679267-7928270.
- 13.A G, S P K, N B, Bs N. (2014) HIV Associated Sensory Neuropathy. J Clin Diagn Res. Jul;8(7):MC04-7. doi: 10.7860/JCDR/2014/8143.4531. Epub 20, 25177587-4149093.
- 14.Cherry C L, Skilasky R L, Lal L. (2006) Antiretroviral use and other risks for HIV-associated neuropathies in an international cohort. Neurology. 66, 867-873.
