Abstract
In the present study, we investigated the chemical compositions, in vitro antioxidant and in vivo hepatoprotective activities of two tea polysaccharides (TPS), which were extracted from two different tea cultivars, Yingshuang (Camellia Senesis, T01) and Yunnan Dayezhong (Camellia Senesis, T09). Compared with T09-TPS, T01-TPS had lower contents of neutral sugar, protein, uronic acid and polyphenol. However, T01-TPS showed stronger scavenging abilities for transient free radicals of hydroxyl radical and superoxide anion radicals and lipid peroxidation inhibition effect, but weaker scavenging ability for stable free radical of DPPH. For hepatoprotective activity in vivo, the results demonstrated that both T01-TPS and T09-TPS could significantly prevent the increase of serum alanine aminotransferase and, aspartate aminotransferase levels, decrease the liver index, reduce the formation of malonydialdehyde and enhance the activities of superoxide dismutase, glutathione peroxidase and peroxidase in carbon tetrachloride-induced liver injury mice. These results suggest that T01-TPS and T09-TPS have potent antioxidant and hepatoprotective activities.
Author Contributions
Academic Editor: Vijay Bharti, DIHAR, DRDO, Ministry of Defence
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Yutao Shi et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Tea is the most widely consumed beverage in the world and also an important agricultural product in some developing countries. Many researches have revealed that tea possesses a wide range of beneficial effects including reduction of cholesterol, depression of hypertension, anti-oxidation and anti-cancer (Ganguly, 2017). These beneficial effects are partly attributed to the chemical ingredients in tea leaves, including polyphenols, polysaccharides, alkaloids and amino acids, etc. (Nie and Xie, 2011; Wang et al., 2012)
Polysaccharides have attracted much attention due to their biological activities in the past two decades. Tea polysaccharides(TPS), one of the main components of tea extracts, have demonstrated beneficial properties in anti-oxidation, reducing blood sugar levels, anti-radiation, anti-blood coagulation, anti-cancer, and hypoglycemic activities(Zhou, Xie & Fu, 2001a; Xie & Nie, 2006; Wang, Wang, & Li, 2001). TPS have active effects on scavenging reactive oxygen species (ROS) including hydroxyl radical, superoxide anion and 1, 1-Diphenyl-2-picryldydrazyl (DPPH) radical in vitro. In addition, a recent report showed that tea polysaccharides extracted from leaf, seed and flower exhibited different antioxidant abilities in vitro(Wang et al., 2012). The cultivar is one of the important factor affecting the components and contents of TPS. Our laboratory have determined the free radical scavenging abilities of TPS extracted from 209 tea cultivars gathered in main tea producing areas in China. The results show that free radical scavenging activities of TPS solely related to the tea cultivars. T01 (Yingshuang)-TPS with the highest free radical scavenging ability and T09 (Yunnan Dayezhong)-TPS with the lowest free radical scavenging ability were screened out respectively.
Liver diseases are a worldwide medical problem because the liver is the principal detoxifying organ and maintains metabolic homeostasis. The liver metabolizes various compounds that produce reactive oxygen radicals (ROS). Oxidative stress can result from an increase in prooxidant formation or a decrease or deficiency in antioxidants. Molecular redox switches and oxygen sensing by the thiol redox proteome and by phosphorylation/dephosphorylation systems are bias involved in signaling, control, and balance redox of a the liver system(Pablo, 2016). In this study, we investigated antioxidant and hepatoprotective activities of T01-TPS and T09-TPS in vitro and in vivo.
Materials and methods
Materials and chemicals
Tea leaves (one bud with three leaves) of Yingshuang (Camellia Senesis, T01) and Yunnan Dayezhong (Camellia Senesis, T09) were picked up from tea plantation in Huazhong Agricultural University. The leaves were fix by steam, dried in an oven, then grounded into powder by a milling machine, passed through 80-mesh sieve and kept in sealed polyethylene bags at -20°C.
2-Deoxyribose, thiobarbituric acid and DPPH purchased from Sigma chemical Co. (MO, USA). Assay kits for superoxide dismutase (SOD), alanine aminotransferase (ALT), aspartate aminotransferase (AST), malonydialdehyde (MDA), glutathione peroxidase (GSH-PX) and Catalase (CAT) were products of Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Carbon tetrachloride (CCl4) and Vitamin C were purchased from Sinopharm Chemical Reagent Co. (Shanghai, China). Bifendate Pills were product of Zhejiang Wanbang pharmaceutical Co. (Zhejiang, China). All other reagents were of analytical grade.
Preparation of TPS
The dried sample powders (100g) were mixed with 2000 mL of distilled water and shaken at 55°C for 2.5 h by electromagnetic stirrer (90-3, Shanghai Huxi Instrument Factory, Shanghai, China), then centrifuged at 4000 rpm for 15 min. The supernatants were concentrated in a rotary evaporator (RE-52AA, Shanghai Yarong Biochemistry Instrument Factory, Shanghai, China). Collected the concentrate, and mixed with three times volume of 95% (v/v) ethanol, stirred vigorously and kept overnight at 4°C. The mixture were centrifugation at 4000 rpm for 15min, collected the precipitates and dried by an vacuum freeze-drier (Coolsafe 110-4, Labogene ScanVac, Lynge, Denmark) (Ni et al., 2004).
Analytical methods of components in TPS
The content of neutral sugar was determined by the anthrone-sulfate method with D-glucose as the criteria ( Dubois, 1956). The uronic acids content was determined according to sulfate-carbazole method with galacturonic acid as the criteria (Bitter and Muir, 1962). Protein was determined by the Coomassie brilliant blue G-250 method with bovine serum albumin as the criteria (Bradford, 1976). Total phenolic content was detected by ferrous tartrate colourimetry method (Yalin Guo, 2016).
TPS antioxidant activity in vitro
Assay of hydroxyl radical scavenging activity
The deoxyribose method to determine the rate of reaction of hydroxyl radical with antioxidant was performed according to the reported method (Zhou et al., 2009). The absorbance of was measured at 532 nm. Vitamin C was used as positive control. All tests performed in triplicate. The capability of scavenging hydroxyl radical was calculated by the following equation
Scavenging ability(%)=A0/A0×100
Where A0 is the absorbance of the control (without samples); A1 is the absorbance of the mixture containing samples; A2 is the absorbance of blank.
IC50 value was determined to be the effective concentration, at which •OH radical was scavenged by 50%. The IC50 value was obtained by interpolation through linear regression analysis.
Assay of superoxide radical scavenging activity
Scavenging effects of TPS on superoxide radicals (O2•-) were assayed by using the xanthine oxidase method. The measurement was finished following the reagent kit. In the PMS-NADH system (Zhou et al., 2009), 1.0 mL NBT, 1.0 mL NADH, 1.0 mL ZSP and 0.4 mL PMS were successively added into test tube, then the reaction mixture was incubated at ambient temperature for 5 min and the absorbance of the mixture solution was determined at 560 nm with the visible spectrometer. Vitamin C was used as positive control, and all tests performed in triplicate. O2•- scavenging activity was calculated according to the following equation:
Scavenging ability(%)=(A0-A1)/A0×100
In the equation, A0 is the absorbance of the control, while A1 is the absorbance of sample.
IC50 value was determined to be the effective concentration, at which O2•- radical was scavenged by 50%. The IC50 value was obtained by interpolation through linear regression analysis.
Assay of DPPH radical scavenging activity
The scavenging effects of samples for DPPH radical were monitored according to the method of the previous report (Duan et al., 2006). Briefly, a 2.0 mL aliquot of test sample (in methanol) was added 2.0 mL of 0.16 mM DPPH methanolic solution. The mixture was vortexed for 1 min and then left to stand at room temperature for 30 min in the dark, and the absorbance read at 517 nm. The ability to scavenge the DPPH radical was calculated using the follow equation:
Scavenging ability (%) = 1 ×100
In this equation, control was DPPH solution without sample, sample was DPPH solution added test sample), and sample blank was only test sample solution without DPPH. Vitamin C was used as positive control. IC50 value was determined to be the effective concentration at which DPPH radical was scavenged by 50%. The IC50 value was obtained by interpolation through linear regression analysis.
Animal experiments
Experimental animals
Male Kunming mice (5 week old) were obtained from The Laboratory Animal Center of Hubei Province (Hubei, China) and housed under controlled temperature (23 ± 2 ℃) with a normal day/night cycle. The animals were fed standard laboratory pellet diet and clean water ad libitum. With the experiment, mice were sacrificed by decapitation. The blood samples were collected and the liver tissues were washed with cold 0.9% saline solution and stored at −80℃ until analysis.
All of the experimental procedures were conducted with the approval of the Institutional Animal Care and Use Committee of the Huazhong Agriculture University and the guidelines for the care and use of laboratory animals in China.
Effects of TPS on hemolysis induced by H2O2 of mice red blood cell
The blood samples were centrifuged at 3000 rpm for 5 min at 4°C to obtain red cell samples. The red cell samples dissolved in the concentration of 0.5% red blood cell suspension with 0.9% NaCl solution. Reaction mixtures contained 1 mL of 0.5% red blood cell suspension, 0.2 mL of TPS samples, and then added 0.1 mL of 100 mM H2O2 to start the reaction. After incubation at 37°C for 1 h, the mixtures centrifuged at 3000 rpm for 5 min; the absorbance of the Supernatant solution measured at 415 nm. 0.5 mg/mL Vitamin C was positive control. Then the inhibition rate calculated as follow:
Inhibition rate (%) = (Acontrol - Asample)/Asample×100 Control is the reaction mixtures added 0.2 mL of pure water instead of TPS; sample is the reaction mixtures.
Lipid peroxidation inhibition assay
The assay performed using the method described by Mee. al. (2001). The livers were homogenized at ratios of 1:9 (w/v) in pre-cooling 0.9% saline solution with a homogenator on ice. The homogenized solution centrifuged at 3000 rpm for 10-15 min. Reaction mixtures contained 0.2 mL of liver homogenized solution, 0.2 mL of TPS sample of certain concentration. After incubation at 37°C for 1 h, the color was developed by adding 2 mL of 10% trichloroacetic acid (TCA) and 2 mL of 0.67% thiobarbituric acid (TBA), the reaction mixtures were then heated in boiling water for 15 min. The absorbance of the resulting solution measured at 532 nm. Then the inhibition rate calculated as follow:
Inhibition rate (%) = (Acontrol - Asample)/Asample×100
Where Acontrol is the absorbance of the control, Asample is the absorbance of sample.
Effects of TPS on mitochondria swelling induced by Vitamin C +Fe2+ in mice liver
50 mL liver homogenized solution centrifuged at 3000 rpm for 10 min. The Supernatant solution centrifuged at 10000 rpm for 10 min. The precipitation was mice liver mitochondrion, and dissolved into 0.5 mg/mL with 0.9% normal saline. 4 mL various concentrations of sample were added to the reaction mixtures, then added 3 mL of 0.5 mg/mL mice liver mitochondrion solution and 0.4 mL of 0.5 mmol/L FeSO4 and 0.4 mL of 0.5 mmo1/L Vitamin C solution. After well mixing, the absorbance of the mixture was measured at 520 nm in 0 min, 20 min, 30 min, 40 min, and the inhibition ability of TPS on the mitochondria swelling was expressed as the following equation:
Inhibition rate (%) = (Acontrol - Asample)/Asample×100. In the equation, Acontrol is the absorbance of the control, and Asample is the absorbance of sample.
Evaluation of hepatoprotective effects of TPS
Animal grouping and experimental design
Animals were raised locked in a room with a controlled temperature of 23±2℃ and a normal day/night cycle. They were allowed free access to basal pellet diet and tap water. After one week of acclimatization, mice were randomly divided into nine groups (n=10). (1) Normal control group (NCG), normal mice were received 0.9% physiological saline solution; (2) CCl4 model control group (MCG), CCl4-induced hepatic injury mice who received 0.9% physiological saline solution; (3) Positive control group (PCG), CCl4-induced hepatic injury mice were received Bifendate Pills at dose of 100 mg/kg body weight; (4)-(6) T01-TPS treated groups (BT), CCl4-induced hepatic injury mice were received T01-TPS at dose of 100, 300, 600 mg/kg body weight; (7)-(9) T09-TPS treated groups (BT), CCl4-induced hepatic injury mice were received T09-TPS at dose of 100, 300, 600 mg/kg body weight. All treatment were administered intragastrically for 4 weeks. After 2 hours of the last administration, animals (except for NCG) were injected intraperitoneally with the 0.10% CCl4 olive oil solution at a dose of 15 mL/kg body weight to induce hepatic injury. NCG was given only olive oil injection. All mice sacrificed at 18 h post-injection of CCl4 or olive oil. The blood samples and livers were collected.
The blood samples were centrifuged at 3000 rpm for 10 min at 4°C to obtain serum samples, and then stored at -20°C. The whole livers weighed and the same part (about 0.5cm×0.5 cm×0.5 cm) of each mouse’s right-middle liver lobule separately fixed in 10% formalin solution for more than 24 h. Then these samples processed by standard histology procedures, embedded in paraffin, cut into thick pieces and mounted on the slide. The liver homogenized in 1:9 volumes of 0.9% NaCl solution. The homogenate centrifuged at 3000 rpm for 10 min, then, the supernatant used as liver total homogenate sample.
Biochemical assay
The liver index calculated according to the formula: Liver index = (mice liver weight/mice weight) ×100%. The activities of ALT and AST and Plasma albumin (ALB) Level in serum, level of MDA, and activities of SOD, GSH-Px and CAT in liver were determined by using commercial reagent kits according to the instruction manuals.
Histologic analysis
The liver samples stained with haematoxylin-eosin (HE) and examined under light microscope (Olympus, Japan) for general histopathology examination. The sectioning and slide preparation of paraformaldehyde-fixed and paraffin-embedded tissues was performed by the core imaging facility of College of Animal science and Technology (HZAU, Wuhan City, Hubei).
Statistical analysis
All data expressed as means ± SD and subjected to One-way analysis (ANOVA) of variance followed by t-test for multiple comparisons. For a single comparison, the significance of differences between the means determined by Student’s t-test. A level of P < 0.01 and P < 0.05 considered statistically significant.
Results
Chemical composition of T01-TPS and T09-TPS
As shown in Table 1. T09-TPS exhibited relatively higher neutral sugar, protein, uronic acid and polyphenol contents than T01-TPS, which probably because T09 was a large-leafed tea cultivar.
Table1. the chemical composition of T01-TPS and T09-TPS| Sample | Neutral sugar (%) | Protein (%) | Uronic acid (%) | Polyphenol (%) |
| T01-TPS | 39.17 | 2.90 | 18.65 | 5.93 |
| T09-TPS | 44.84 | 3.73 | 23.73 | 8.56 |
Antioxidant activities in vitro of T01-TPS and T09-TPS
Effects of TPS on hydroxyl radical, superoxide anion radical and DPPH radical
As shown in Figure 1A, the IC50 value of T01-TPS, T09-TPS and Vitamin C were of 1.14 mg/mL, 2.47 mg/mL and 13.10 mg/mL, respectively. The scavenging activities of T01-TPS and T09-TPS was stronger than that of Vitamin C.
Figure 1A.Hydroxyl radical scavenging abilities of samples
The scavenging activities of T01-TPS, T09-TPS and Vitamin C for superoxide anion radical(Figure 1B), the IC50 were 0.68 mg/mL, 2.94 mg/mL, 0.09 mg/mL, separately. T01-TPS and T09-TPS exhibited relatively weaker scavenging activity than Vitamin C. For all the three samples, the scavenging effects for superoxide anion radical were in a concentration-dependent manner.
Figure 1B.Superoxide anion radical scavenging abilities of samples
Figure 1C indicated that the scavenging effects of T01-TPS, T09-TPS and Vc for DPPH radical raised by increasing concentration, with IC50 value of 0.18 mg/mL, 0.09 mg/mL, 0.007 mg/mL, respectively. The results suggested that scavenging activity of T09-TPS for DPPH radical was stronger than T01-TPS, but both of T01-TPS and T09-TPS were weaker than Vitamin C.
Effects of TPS on hemolysis induced by H2O2 of mice red blood cell
In Figure 2, T01-TPS, T09-TPS and Vitamin C had inhibition to the hemolysis of red blood cells induced by H2O2, and the inhibition effects increased with the rising of sample concentration. The inhibition ability of T01-TPS was better than that of T09-TPS, but both of them were lower than Vitamin C. The results demonstrated that T01-TPS and T09-TPS possessed moderate inhibition ability of hemolysis induced by H2O2 of mice red blood cell.
Figure 2.Inhibition effects of samples on hemolysis induced by H2O2 of mice red blood cell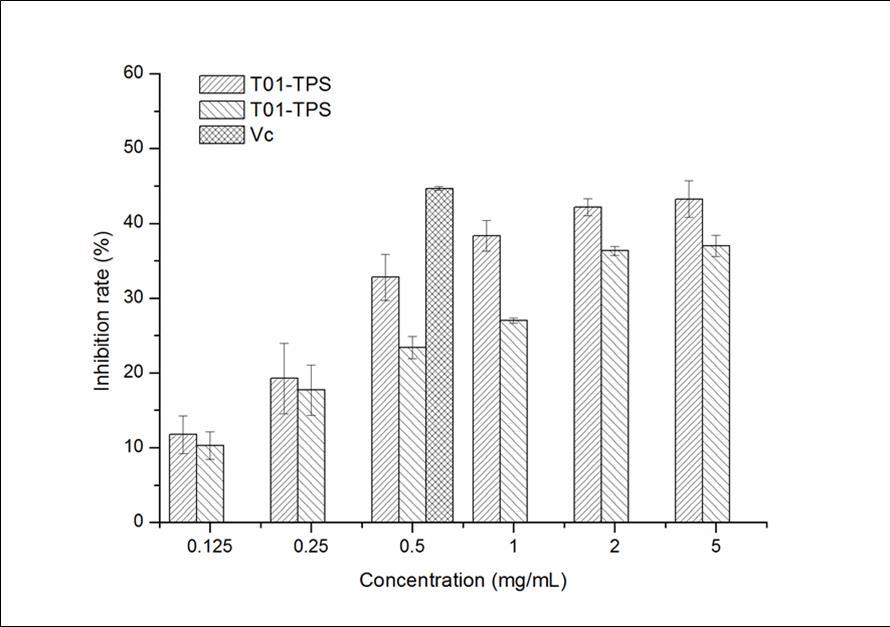
Effects of TPS on lipid peroxidation inhibition
Both of T01-TPS and T09-TPS could reduce MDA production from liver homogenate Figure 3.The inhibition rates of T09-TPS, T01-TPS was 73.48% and 75.94% when its concentration was 62.5μg/ml. However, when Vitamin C concentration was 31.2μg/ml, it had stimulating effect on MDA production.
Figure 3.Inhibition effect of samples on liver lipid peroxidation in mice
Effects of TPS on mitochondria swelling induced by Vc +Fe2+ in mice liver
The results of inhibition effects of T01-TPS and T09-TPS on mouse liver mitochondria swelling as shown in Figure 4Figure 5. and Figure 5. The decline of A520 of the control group indicates that the damage of mitochondrial oxidative accelerate with inducer. A520 of the sample group declines relatively slower with a certain concentration of T01-TPS and T09-TPS, which demonstrated that both T01-TPS and T09-TPS have a certain inhibition ability of mouse liver mitochondrial swelling. The inhibition ability increased with increasing concentration. At the same concentration, the inhibitory effect of T01-TPS is stronger than T09-TPS, at a concentration of 2.0 mg/mL, the inhibition rate of T01-TPS and T09-TPS were 64.92% and 59.19%.
Figure 4.Inhibition effect of TPS on mitochondria swelling induced by VC+Fe2+a
Figure 5.Inhibition effect of TPS on mitochondria swelling induced by VC+Fe2+ (2)
In vivo hepatoprotective and antioxidant effects assay
Effects of TPS on liver index in mice
As shown in Table 2. compared with NCG, the liver index of MCG was significantly increased (P<0.01), suggesting injection of CC14 caused acute liver injury, while T01-TPS (100, 300, 600mg/kg) and T09-TPS (100, 300, 600mg/kg) was more or less decreased as compared with MCG, but not significant. The liver index of PCG (Bifendate) was obviously decreased as compared to MCG (P<0.01).
Table 2. Effects of TPS on liver index of CC14-treated mice| Groups | Liver Index |
| NCG(Normal control group) | 3.81±0.20 |
| MCG(CCl4 model group) | 4.96±0.63A |
| PCG(Bifendate, 100 mg/kg) | 4.26±0.39a, B |
| T01-TPS (100 mg/kg) | 4.80±0.31A, c |
| T01-TPS (300 mg/kg) | 4.58±0.52A |
| T01-TPS (600 mg/kg) | 4.78±0.23A, c |
| T09-TPS (100 mg/kg) | 4.87±0.66A, c |
| T09-TPS (300 mg/kg) | 4.71±0.66A |
| T09-TPS (600 mg/kg) | 4.56±0.18A |
Effects of TPS on ALT and AST activities and ALB content in serum
Effects of TPS on serum ALT and AST activities and ALB level in CC14-treated mice presented in Table 3. Only administration of low dose (100mg/kg) of T01-TPS did not change ALT and ALB level in the serum significantly. Low dose (100mg/kg) of T09-TPS did not cause significant changes in the serum ALT level. While administration of higher dose (300mg/kg, 600mg/kg) of T01-TPS and T09-TPS (100, 300, 600mg/kg), serum ALT and AST decreased in CC14-treated mice compared to MCG. Meanwhile, the ALB level in serum obviously increased in T01-TPS (300, 600mg/kg) (P<0.05, P<0.01) and T09-TPS (100, 300, 600mg/kg) (P<0.05, P<0.05, P<0.01) compared with NCG. These data suggest that both T01-TPS and T09-TPS effectively protect liver function against CCl4-induced hepatotoxicity.
Table 3. Effects of TPS on serum ALT and AST activities in CC14-treated mice| Groups | ALT(U/L) | AST(U/L) | ALB(g/L) |
| NCG (Normal control group) | 87.74±8.45 | 116.97±11.92 | 40.43±2.99 |
| MCG (CCl4 model group) | 256.92±26.75 A | 376.33±16.35 A | 35.64±2.99 A |
| PCG (Bifendate, 100 mg/kg) | 176.28±15.96 A, B | 313.04±10.08 A, B | 41.49±4.47 B |
| T01-TPS (100 mg/kg) | 214.23±16.51 A | 337.26±16.76 A, b | 38.51±1.04 a, c |
| T01-TPS (300 mg/kg) | 196.44±8.81 A, b | 327.83±18.36 A, B | 39.30±2.61 b |
| T01-TPS (600 mg/kg) | 184.66±16.65 A, B | 318.47±15.25 A, B | 41.36±2.58 B |
| T09-TPS (100 mg/kg) | 218.49±12.42 A | 343.89±13.16 A, b | 38.89±4.69 b |
| T09-TPS (300 mg/kg) | 204.41±8.55 A, b | 332.17±14.09 A, B | 39.24±3.66b |
| T09-TPS (600 mg/kg) | 189.25±11.45 A, B | 330.54±7.57 A, B | 40.93±2.77 B |
Effects of TPS on SOD, GSH-Px and CAT activities in mice liver
As shown in Table 4, compared to NCG, treatment of CC14 significantly increased the MDA content and decreased the level/activity of SOD, GSH-Px and CAT, suggesting stronger oxidative stress and lipid peroxidation in liver tissue. Compared to MCG, treatment of T01-TPS (300, 600mg/kg) (P<0.01) and T09-TPS (300, 600mg/kg) (P<0.05) significantly inhibited the MDA formation. Treatment of T01-TPS (P<0.01) and T09-TPS (P<0.05) markedly increased the SOD activity at all the three tested concentrations. GSH-Px activities were significantly increased in groups treated with both of the higher dose (300, 600mg/kg) of T01-TPS (P<0.01) and T09-TPS (P<0.01) compared to that of MCG. CAT activities were obviously increased in groups treated with T01-TPS (100, 300, 600mg/kg) (P<0.01, P<0.01, P<0.05) and T09-TPS (100, 300, 600mg/kg) (P<0.05, P<0.05, P<0.01) compared with MCG.
Table 4. Effects of TPS on MDA, SOD, GSH-Px and CAT activities in CC14-treated mice liver| Groups | MDA (nmol/mgprot) | SOD (U/L) | GSH-Px(U/mgprot) | CAT(U/gprot) |
| NCG(Normal control group) | 4.66±0.75 | 95.19±9.61 | 236.17±41.81 | 54.65±3.05 |
| MCG(CCl4 model group) | 7.84±2.37A | 73.47±7.18a | 171.01±23.50A | 32.10±10.79A |
| PCG(Bifendate, 100 mg/kg) | 6.21±1.28a, b | 105.80±8.74B | 234.73±53.77B | 44.47±11.19b |
| T01-TPS (100 mg/kg) | 6.64±1.94a | 108.28±11.56B | 253.72±40.07B | 46.67±9.43B |
| T01-TPS (300 mg/kg) | 5.75±1.15B | 121.50±12.95A, B | 257.35±53.13B | 50.80±10.25B |
| T01-TPS (600 mg/kg) | 5.49±0.87B | 81.57±8.48b, C | 215.16±44.96a | 42.29±9.00b |
| T09-TPS (100 mg/kg) | 6.87±1.52A | 83.52±13.67b, C | 204.80±32.97a | 43.57±2.45b |
| T09-TPS (300 mg/kg) | 6.27±1.34a, b | 85.48±10.45b, c | 242.14±63.37B | 44.34±14.31b |
| T09-TPS (600 mg/kg) | 6.16±1.03a, b | 80.51±8.49b, C | 196.38±29.69B | 48.04±12.29B |
Histopathological results
The effects of T01-TPS and T09-TPS and Bifendate on liver histopathology of CCl4-treated mice shown in Figure 6. Liver sections from the normal control group (Fig. 6A) showed normal hepatic cells with well-preserved cytoplasm, prominent nucleus and clear central vein, while massive inflammation and infiltraton were observed around central vein in the CCl4-treated mice in model control group (Fig. 6B), and many ballooned hepatocytes were also found. For the positive control group, Bifendate exhibited a significant protective effect evidenced by without eident inflammation zones or color change of any hepatocytes in the liver sections (Fig. 6C). Mice pretreated with T01-TPS and T09-TPS exhibited marked improvements in the liver histopathology in CCl4-treated mice (Fig. 6D-H). In particular, administration of T01-TPS (300, 600mg/kg) resulted in almost complete normalization of the tissues, while only very mild injury observed.
Figure 6.Effect of T01-TPS (100, 300, 600mg/kg), T09-TPS (100, 300, 600mg/kg) and Bifendate on liver histopathology of CCl4 treated mice (HE×200). (A) liver section of NCG(normal control group); (B) liver section of MCG(CCl4 model group); (B) liver section of PCG(Bifendate( 100 mg/kg) + CCl4); (D-F) liver section of T01-TPS(100,300, 600mg/kg) + CCl4 group; (G-I) liver section of T09-TPS(100,300, 600mg/kg) + CCl4 group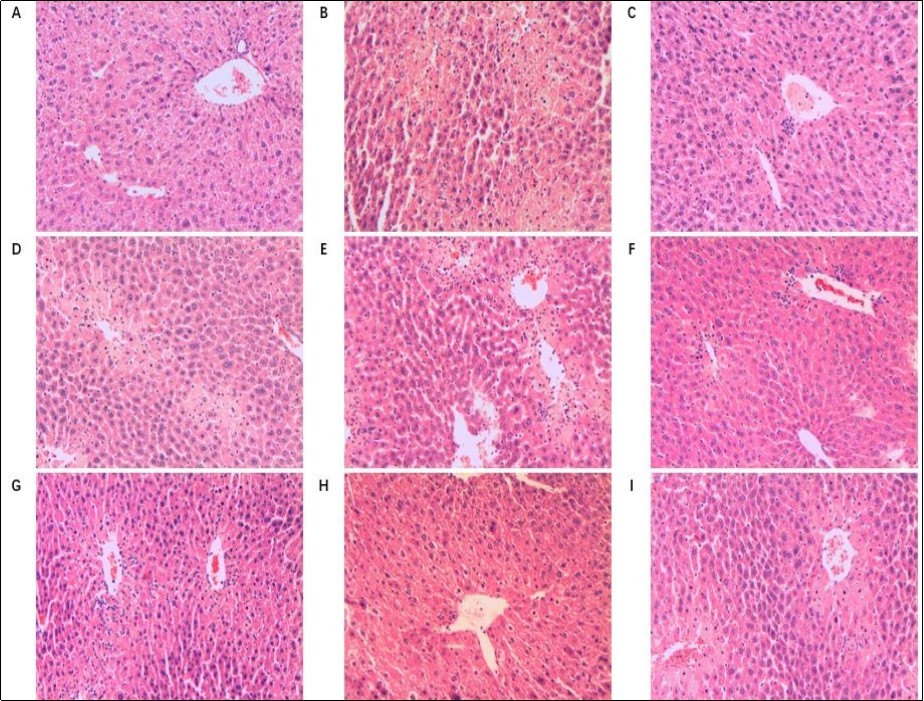
Discussion
A vast number of evidence has implicated that oxidative stress is associated with a wide range of diseases (Finkel & Holbrook, 2000). Thus, the antioxidant activity of bioactive compounds from various sources can considered as an important index by which their potential benefit for human health will be evaluated.
Hydroxyl radicals have the strongest chemical activity among various reactive oxyen species. They can damage a wide range of essential biomoleccules such as amino acid, protein, and DNA (Halliwell and Gutteridge, 1990). However, there is no specific enzyme to defense against hydroxyl radicals in human body. Therefore, it would be of great significance to discover some compounds with good hydroxyl radical scavenging activity for the oxidative stress induced diseases (Zhou et al., 2010). Studies have shown that polysaccharides with hydroxyl radicals scavenging ability have the same structure feature that have one or more alcohol or hydroxyl groups, and the scavenging capability is related to the amount of these hydroxyl active groups (Yang et al., 2010). In the present study, addition of the T01-TPS and T09-TPS decreased the formation of MDA remarkable. It figured that TPS could compete with D-deoxyribose and thereby scavenge hydroxyl radical. There might be two mechanisms: Firstly, TPS is a group of monosaccharide held together by chemical bonds, and each monosaccharide consists of variable number of active hydrogens, and these active hydrogens combine with hydroxyl radical to form stable complex water. Secondly, TPS was a complex with hydroxyls and carboxyl, which can complex with Fe2+ and Cu2+ to inhibit the production of ·OH, and then affects the start of lipid peroxidation to inhibit the producing of reactive oxygen.
Superoxide radical is a very important radical in biological systems, it can cause oxidizing damage of biological macromolecular, induction of lipid peroxidation which leads to a decrease in the fluidity of the membrane, and then cause diseases and old age (Inal et al., 2001). In the PMS/NADH-NBT system, the reduction of NBT with NADH mediated by PMS under aerobic conditions inhibited upon addition of antioxidants. The addition of PMS to this system provoked the reduction, and the reduction of NBT by O2- occurring in the re-oxidation of reduced PMS with O2 (Wang et al., 2012). Antioxidants would lower the steady-state concentration of NBTH·by scavenging O2- and therefore decreased the rate of production of the formazan by reaction. In the present study, the decrease of absorbance at 560 nm after addition of T01-TPS and T09-TPS indicated the consumption of the generated superoxide anion in the reaction mixture, which positively correlated to the superoxide anion scavenging activity. It showed the inhibitory effect of T01-TPS and T09-TPS on superoxide radical.
The DPPH radical is a stable organic free radical with absorption maximum band around 515-528 nm, it is a useful reagent for investigating free radical scavenging activities of antioxidant materials. Antioxidants transfer either electrons or hydrogen atoms to DPPH and thus reduce a number of DPPH radical equal to their number of available hydroxyl groups. In addition, the stable yellow-colored diphenylpicryl hydrazine (DPPH-H) simultaneously formed, and the extent of the reaction will depend on the hydrogen donating ability of the antioxidants. (Alma et al., 2003; Karioti et al., 2004).But unlike the D-deoxyribose-iron system and xanthine oxidase method , DPPH method provides a message of antioxidants reacting with stable free radical instead of transient free radicals. In present study, the result suggest that T01-TPS has stronger scavenging ability of transient free radicals than T09-TPS, but it also show opposite result in scavenging stable free radical DPPH. The mechanism needs to further study. However, we could speculate that polysaccharides from different tea cultivars scavenge free radicals in different manners.
Lipid peroxidation is an important risk factor in the oxidative damage. Peroxidation MDA can reflect the extent of oxidation, increase of membrane permeability, which may cause the material easily go to the other side through the membrane, resulting in oxidative hemolysis of erythrocyte and mitochondrial swelling. The results show that T01-TPS and T09-TPS samples can inhibit oxidative hemolysis of erythrocyte in vitro induced by H2O2 and inhibit MDA formation of liver homogenates and mitochondrial swelling. This indicates that tea polysaccharide molecular can capture reactive oxygen species, which generate in the lipid oxidation chain reaction and block or slow down the lipid peroxidation.
CCl4 induced hepatotoxicity is the common experimental model for the hepatoprotective drug screening. CCl4 can be metabolized to the trichloromethyl radical (•CCl3) and proxy trichloromethyl radical (•OOCCl3) by cytochrome P450 2E1 enzyme (Jia et al., 2011). These radicals bind covalently to the macromolecules and cause peroxidative degradation of cellular lipid membrane, which will cause the loss of integrity of cell membranes, and the necrosis of hepatocytes (Ranawat et al., 2010). Liver damage can assessed by biochemical studies. AST and ALT levels most frequently used in the diagnosis and management of liver diseases. AST and ALT are present in high concentration in hepatocytes, while they leak into the circulation when hepatocytes or their membranes are damaged (Kew, 2000). MDA level in liver tissue is used to reflect the extent of lipid peroxidation in hepatocytes, as MDA is one of the end-products of polyunsaturated fatty acid peroxidation (Esterbauer et al., 1991). Hepatic GSH plays a crucial role in scavenging ROS and maintaining enzymatic antioxidant as an important non-enzymatic antioxidant. In the CCl4 treated mice GSH plays a key role in eliminating the reactive toxic metabolites of CCl4 (Ou et al., 2010). SOD is one of the major antioxidant enzymes responsible for the defense against potentially free radicals that cause oxidative stress, but it is highly sensitive to and easily inactivated by lipid peroxide and ROS. The injection of CCl4 will cause liver damage, led to the elevation of AST and ALT levels in serum and MDA level in liver tissue, while decreasing of SOD, GSH-Px and CAT activities. Pretreatment with both T01-TPS and T09-TPS extracted from different tea cultivar Yingshuang (Camellia Senesis) and Yunnan Dayezhong (Camellia Senesis) have reversed these trends towards normalizations, reflecting that T01-TPS and T09-TPS possesses potent hepatoprotective activity in vivo, which might attribute to their strong antioxidant activity. Histopathological observation of the liver section also supports this conclusion.
Lots of evidence has suggested that the bioactivity of tea polysaccharide is associated with its physiochemical and structural features, especially the advanced structure. The bioactivity changes with the shift of the structure. Two tea polysaccharides, NTPS and ATPS, had remarkable hypoglycemic activities. After sulfuric etherification, the hypoglycemic activity of NPTS improved greatly, but that of ATPS did not change markedly (Wang & Jin, 2005). A previous study in our laboratory also revealed that the micro-morphology, conformation and the behavior of aggregation of OTPS was changed after fermentation, meanwhile, the bioactivity was largely improved (Ni et al., 2004). Our results indicated that T01-TPS possessed stronger antioxidant activity than T09-TPS, this may due to their physiochemical and structural features. Future studies on the structure-activity relationship of TPS were in progression.
Conclusion
T01-TPS extracted from small-leafed tea cultivar shows relatively stronger ability in scavenging hydroxyl radical and superoxide radical, inhibiting lipid peroxidation and protecting liver injury than T09-TPS extracted from large-leafed tea variety, which suggested that tea cultivar played an important role in the activity of TPS. The order of antioxidant and hepatoprotective activity in vivo of T01-TPS and T09-TPS were similar to free radical scavenging activity in vitro, which suggesting that the determination of free radical scavenging activity in vitro may be an effective way in functional tea variety selecting instead of determining both in vitro and in vivo. Moreover, T01-TPS had lower activity for scavenging stable free radical DPPH, but it had a higher activity for scavenging hydroxyl radical and superoxide radical, which demonstrated that polysaccharides from different tea cultivars had a different mechanism in scavenging different kinds of free radicals. Further work is under way to characterize the structure-activity relationship of polysaccharides from different tea cultivars, aiming to promote bioactive tea polysaccharides screening and its utilization.
Acknowledgments
Funding:
This work was supported by National Nature Science Funds of China (No. 31000316), A-type Project of Department of Education of Fujian Province (No.JA11026) and Research Funds for Young Teachers of Wuyi University (No.XQ201014). . The funders did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The contents of this work are solely the responsibility of the authors.
Shiqi Zhou, Associate professor of college of Animal Science and Technology, conducted most of histopathological works. He also guided us to collect mice blood and tissue samples. We appreciate his contribution to this project.
References
- 1.Ganguly Subha. (2017) Medicinal and health related benefits of Green Tea for human intake. , Annals of Plant Sciences 6, 1604-1605.
- 2.Bolling B W, Chen C O, Blumberg J B. (2009) Tea and health: preventive and therapeutic usefulness in the elderly Curr. , Opin. Clin. Nutr. Metab. Care 12, 42-48.
- 3.Ni D J, Chen Y Q, Xie B J, Zhang Y, Zhou J R. (2004) . Spectrum, Morphological and Thermal Characteristics of OTPS 2-1 in Polysaccharides from Oolong Tea. Chem J Chin Univ 25(12), 2263-2268.
- 4.Bradford Marion M. (1976) A rapid method the quantities utilizing binding. Analytical Biochemistry.72. 248-254.
- 5.Ganguly Subha. (2017) Medicinal and health related benefits of Green Tea for human intake. , Annals of Plant Sciences 6, 1604-1605.
- 7.Guo Yaling, Lai.Lingling Analysis on Quality Indicators of Taste and Infusion Color and. Discrimination of Famous and Superior Green Tea with Different Appearances in Fujian Province, PR China, 2015 .Journal of Food Processing and Preservation2084-1092 .
- 8.Alma M H, Mavi A, Yildirim A, Digrak M, Hirata T. (2003) Screening chemical composition and in vitro antioxidant and antimicrobial activities of the essential oils from Origanum syriacum L. growing in turkey. , Biological & Pharmaceutical Bulletin 26.
- 10.Duan X-J, Zhang W-W, Li X-M, Wang B-G. (2006) Evaluation of antioxidant property of extract and fractions obtained from a red alga, Polysiphonia urceolata. , Food Chemistry 95, 37-43.
- 11.Inal M E, Kanbak G, Sunal E. (2001) Antioxidant enzyme activities and malondialdehyde levels related to aging. , Clinica Chimica Acta 305.
- 12.K D M G. (1956) Colorimetric method for determination of sugars and related substances. , Analytical Chemistry 28, 350-353.
- 13.Karioti A, Hadjipavlou-Litina D, Mensah M L K, Fleischer T C, Skaltsa H. (2004) Composition and antioxidant activity of the essential oils of Xylopia aethiopica. , Journal of Agricultural and Food Chemistry 52.
- 14.Ni D-J, Chen Y-Q, Xie B-J, Zhang Y, Zhou J-R. (2004) . Spectrum, Morphological and Thermal Characteristics of OTPS 2-1 in Polysaccharides from Oolong Tea. Chemical Jounal of Chinese Universities 25.
- 15.Nie S-P, Xie M-Y. (2011) A review on the isolation and structure of tea polysaccharides and their bioactivities. , Food Hydrocolloids 25, 144-149.
- 16.Ueda J, Saito N, Shimazu Y, Ozawa T. (1996) A comparison of scavenging abilities of antioxidants against hydroxyl radicals. Archives of biochemistry and biophysics 333. 377-384.
- 17.Wang Y, Mao F, Wei X. (2012) Characterization and antioxidant activities of polysaccharides from leaves, flowers and seeds of green tea. , Carbohydrate polymers 88, 146-153.
- 18.Zhou D, Chen Y, Ni D. (2009) Effect of water quality on the nutritional components and antioxidant activity of green tea extracts. , Food Chemistry 113, 110-114.
- 19.Alma M H, Mavi A. (2003) Screening chemical composition and in vitro antioxidant and antimicrobial activities of the essential oils from Origanum syriacum L. growing in turkey. , Biological & Pharmaceutical Bulletin 26(12).
- 21.Chen H, Xie B. (2002) The Preventive and Curative Effects on Diabetic Mice of Tea Polysaccharides. , Acta Nutrimenta Sinica 24(1), 85-86.
- 22.Chen Y, Yu Z. (2005) Effect of Tea Cultivars and Tenderness on Tea Polysaccharide. , Journal of Huazhong Agricultural University 24(4).
- 23.Cheung L M, Cheung P C K. (2003) Antioxidant activity and total phenolics of edible mushroom extracts. , Food Chemistry 81(2), 249-255.
- 24.Gui-peng F. (2006) The Method of Polysaccharide′s Structure Research and Its Development of Activity Research. , Journal of Pingyuan University 23(5), 128-130.
- 25.Inal M E, Kanbak G. (2001) Antioxidant enzyme activities and malondialdehyde levels related to aging. , Clinica Chimica Acta 305-1.
- 26.Dubois M, Gilles K A, Hamilton J K, Rebers P A, Smith F. (1956) Colorimetric method for determination of sugars and related substances. , Analytical Chemistry 28(3), 350-353.
- 27.Karioti A, Hadjipavlou-Litina D. (2004) Composition and antioxidant activity of the essential oils of Xylopia aethiopica. , Journal of Agricultural and Food Chemistry 52(26).
- 28.Kaur C, Kapoor H C. (2001) Antioxidants in fruits and vegetables: The millennium's health. , International Journal of Food Science and Technology 36(7).
- 29.Lei L I, Dongfeng W. (2006) Study on Food Functionality of Tea Polysaccharides. , Journal of tea science 26(2).
- 30.Li B, Wang Z-Z. (2000) Cloning, expression and characterization of a cDNA (6A8) encoding a novel human alpha-mannosidase. , European Journal of Biochemistry 267(24).
- 31.Liu F, Ooi V E. (1997) Free radical scavenging activities of mushroom polysaccharide extracts. , Life sciences 60(10), 763-771.
- 32.Ni D, Chen Y. (2003) Effect of Oolong Tea Polysaccharide on Hepatic-nephritic Antioxidation and Histommorphology in the Diabetic Rats. , Journal of tea science 23(1), 11-15.
- 33.Ni D, Xie B. (2003) Conditions and parameters for extraction of tea polysaccharides. , Transactions of the Chinese Society of Agricultural Engineering 19(2), 176-179.
- 34.Pang Z J, Chen Y. (2000) Effect of polysaccharide krestin on glutathione peroxidase gene expression in mouse peritoneal macrophages. , British Journal of Biomedical Science 57(2), 130-136.
- 35.Shaoping N I E, Mingyong X I E. (2006) Antioxidative Activity Evaluation Study on Tea Polysaccharide by Scavenging DPPH. , Food Science 27(3), 34-36.
- 36.Tsai M-C, Song T-Y. (2007) Antioxidant properties of water-soluble polysaccharides from Antrodia cinnamomea in submerged culture. , Food Chemistry 104(3), 1115-1122.
- 37.Tsiapali E, Whaley S. (2001) Glucans exhibit weak antioxidant activity, but stimulate macrophage free radical activity. , Free Radical Biology and Medicine 30(4), 393-402.
- 38.Ueda J, Saito N. (1996) A comparison of scavenging abilities of antioxidants against hydroxyl radicals. , Archives of biochemistry and biophysics 333(2), 377-384.
Cited by (1)
- 1.Salehi Mohammad, Rashidinejad Ali, 2025, Multifaceted roles of plant-derived bioactive polysaccharides: A review of their biological functions, delivery, bioavailability, and applications within the food and pharmaceutical sectors, International Journal of Biological Macromolecules, 290(), 138855, 10.1016/j.ijbiomac.2024.138855

