Abstract
The use of herbal medicine is becoming popular day by day due to toxicity and side effects of allopathic medicines. Considering the medicinal importance of the plant Murraya koenigii Linn (Curry leaf), the antioxidant activity (AC), total phenolic content (TPC), total flavonoid content (TFC), of different fractions of methanolic extract (DIA-ion resin adsorbed fraction, chloroform, Ethyl acetate and petroleum ether) of M. koenigii were investigated. Among the fractions, DIA-ion resin-adsorbed fraction showed the highest total antioxidant activity with absorbance 2.320±06 and petroleum ether fraction showed the lowest total antioxidant activity with absorbance 1.944 at 100 mg/ml concentration. The TPC were found range between 13.285 to 17.52 mg GAE/g while the highest amount of TFC recorded among the extracts was 16.65 mg CatE/g. DPPH free radical scavenging activity of different extracts of leaves was also measured where DIA-ion resin-adsorbed fraction had the highest free radical scavenging activity with IC50 value 15.53 µg/ml. In the present study phenolic compound were found to be the predominant components in the leaves of M. koenigii indicating that they are potent antioxidant.
Author Contributions
Academic Editor: Jie Yin, Institute of Subtropical Agriculture and University of Chinese Academy of Sciences
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Ismat Ara Dahlia, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Plants which, form the backbone of traditional medicines, have been used for thousands of years as a source of invaluable bioactive compounds1. In most Asian countries, herbal products play an important role to treat wounds, burns, intestinal problems, coughs, and general torpor2. The use of plants as traditional remedies, and to treat burns and wounds is an important aspect of health management and is also an efficient way to promote cheaper health care options3,4. Many researchers have reported in vitro and in vivo evidence to support various plant materials as topical anti-microbial agents to enhance wound healing5. Several indigenous plants and formulations for managing cuts, bruises, burns and wounds have been described in folkloric as well as the Ayurvedic system of medicine6,7. In Bangladesh 5,000 species of angiosperm are reported to occur8. The numbers of medicinal plants included in the ‘materiamedica’ of traditional medicine in this subcontinent now stand at about 2000. More than 500 of such medicinal plants have so far been enlisted as growing in Bangladesh9. Dhaka, Rajshahi, Shylet and Chittagong division is rich in medicinal plants8. Bangladesh is situated at the complex interface of the Indian, Himalayan and Southeast Asian biographic regions, and historically it is well-endowed with very diverse complements of terrestrial and aquatic flora and fauna that include a considerable number of medicinal plant resources10.
Murrayakoenigii, commonly known as curry leaf or Kari Patta in Indian dialects, belongs to the family Rutaceae, which represents more than 150 genera and 1600 species. Murraya comprises of about 11 species of shrubs, and small trees. Its distribution ranges encompass the tropical and subtropical regions of Sri Lanka, India, South China to South-East Asia, Malaysia, New Guinea, North-East Australia, and New Caledonia11. Two of such plants namely M. Koenigii (L.) Spreng and M.Paniculata (L.) Jack, were reported from India. The latter commonly called ‘orange jasmine’ is grown mainly as an ornamental in Jammu and Kashmir. However, M. Koenigii is a semi-deciduous aromatic shrub or tree-let with culinary and medicinal properties12. The leaves are aromatic which contain proteins, carbohydrates, fiber, minerals, carotene, nicotinic acid, and vitamin C, and are also rich in vitamin A and calcium. They also contain high amount of oxalic acid, crystalline glycosides, carbazole alkaloids, koenigin and resin. Fresh leaves contain 2.5% yellow color, volatile oil13. Fresh leaves, dried leaf powder and essential oil are widely used for flavorings soups, curries, fish and meat dishes, eggs dishes, traditional curry powder blends, seasoning and other food preparations have a versatile role to play in traditional medicine and also in soap and cosmetic aromatherapy industry14. Recent studies found some compound include the alkaloids,mahanimbine15, girinimbine16, numerous carbazole alkaloids17, and cinnamaldehyde18 in curry tree leaves, stems, and seeds. It was also reported that the fresh leaves of M. koenigii possesses anti-microbial, mosquitocidal, topo-isomerase inhibition, and antioxidant properties19. However, the aims of this study is to determine total phenols, flavonoids and the antioxidant potential of M. koenigii leaves extracts with different solvents.
Materials and Methods
Plant Material and Sample Preparation
M. koenigii (plant) leaves were collected from the adjacent areas of BCSIR Laboratories Rajshahi, and Botanical Garden, University of Rajshahi. The collected materials were washed thoroughly in water, chopped, air dried for a week at 35-40oC and pulverized in electric grinder. Dried ground leaves of Murrayakoenigiiwere exhaustively extracted with methanol (MeOH, Analytical Grade, BDH Laboratory Supplies) in soxhlet apparatus. The resulting juicy extract was filtered through Whatman paper No.1 and concentrated under reduced pressure at 45°C using rotary evaporator to obtain a crude residue (23.5%).Then water triturate part was collected from crude extract. The water triturate fraction was passed through a previously well packed dia-ion resin column which has selectivity to collect only the phenolic group containing compounds. Then the materials, which were bound in resin column, were collected by passing methanol solvent. Then Petroleum ether, Ethyl acetate and Chloroform solvents were passed through the residue respectively. Finally petroleum ether, Ethyl acetate and Chloroform triturate were collected. The extracts thus obtained were kept in refrigerator until further analysis.
Chemicals and Reagents
All chemicals used were of analytical grade. 1,1-Diphenyl-2- picrylhydrazyl (DPPH), Ascorbic acid, catechin, BHT, gallic acid, anhydrous sodium carbonate (Na2CO3), aluminum trichloride, potassium acetate, sodium acetate, ferric chloride hexahydrate (FeCl3.6H2O), Folin–Ciocalteu reagent were purchased from Sigma–Aldrich. All chemicals and reagents were used without further purification.
Determination of Total Phenolic Content
Total phenolic content of different extractives of Murrayakoenigii were determined by adopting the method20 involving Folin-Ciocalteu reagent as oxidizing agent and gallic acid as standard. An aliquot from each of the plant extract (0.5 ml) and standard gallic acid solution (100, 200, 300, 400, and 500 μg/ml) was added with 2.5 ml of Folin–Ciocalteu (Diluted 10 times with water) reagent and 2.5 ml of Sodium carbonate (7.5%) solution. The test tube was incubated for 20 minutes at 25oC to complete the reaction. A typical blank solution containing all reagents except plant extract or standard was also prepared. Then the absorbance of the solution was measured at 760 nm using a spectrophotometer against blank. Total phenolic contents of extracts were expressed as mg gallic acid equivalent (GAE)/g dried extract. All samples were analyzed in triplicate.
Determination of Total Flavonoids Content
Total flavonoid content was determined using the aluminum colorimetric method 21,22 with some modifications using catechin as the standard. A calibration curve of catechin was prepared in the range of 0–200 lg/ mL. Briefly, extract (0.5 mL) and standard (0.5 mL) were placed in different test tubes and to each 10% aluminum chloride (0.1 mL), 1 M potassium acetate (0.1 mL), 80% methanol (1.5 mL) and distilled water (2.8 mL) were added and mixed. A blank was prepared in the same manner where 0.5 mL of distilled water was used instead of the sample or standard, and the amount of aluminum chloride was also replaced by distilled water. All tubes were incubated at room temperature for 30 min. The absorbance was taken at 415 nm. The concentration of flavonoid was expressed as mg catechin equivalent (CatE) per gram of extract.
Determination of Reducing Power Capacity
Solutions of various concentrations (5, 10, 20, 40 and 80 μg/ml) were prepared in corresponding solvents with each type of extractive of M. koenigii. This mixture was kept at 50ºC in water bath for 20 minutes. After cooling, 2.5 ml of 10% trichloro acetic acid was added and centrifuged at 3000 rpm for 10 min. The upper layer of solution (2.5 ml) was mixed with distilled water (2.5 ml) and a freshly prepared ferric chloride solution (0.5 ml). Control was prepared in similar manner excluding samples. The absorbance was measured at 700 nm. Ascorbic acid at various concentrations was used as standard. Increased absorbance of the reaction mixture indicated the increase in reducing power.
Determination of Total Antioxidant Activity
Total antioxidant activity of different extracts of M. koenigiiwas determined with some modifications23. Solutions of various concentrations (5, 10, 20, 40 and 80 μg/ml) were prepared in corresponding solvents with each type of extractive of M. koenigii, and standard substance (Ascorbic acid) and mixed with 3 ml of reaction mixture containing 0.6 M sulphuric acid, 28 mM sodium phosphate and 1% ammonium molybdate. The mixtures were incubated at 95oC for 10 minutes to complete the reaction. Then the absorbance was measured at 695 nm.
Determination of DPPH Radical Scavenging Activity
The antioxidant activity of M. koenigii extracts were measured in comparison to standard antioxidant ascorbic acid (BDH, England) depending on the scavenging effect of 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical. The whole procedure was performed according to procedure described by Bracae et al. (2001)24. Briefly, different concentrations (10, 20, 40, 80, and 100μg/ml in methanol) of ascorbic acid solution(1ml) as well as M. koenigii extracts solution (1ml) were mixed with 3 ml of 0.4 mM DPPH solution. The mixtures were kept in dark for 30 minutes to measure the absorbance at 517 nm using GENESYSTM 20 Spectrophotometer (Thermo Spectronic, USA). The scavenging activity against DPPH was calculated using the equation:
Scavenging activity (%) = ((A - B) / A) x 100
Where A is absorbance of control (DPPH solution without the sample), B is the absorbance of DPPH solution in the presence of the sample (extract/ ascorbic acid).
Results and Discussion
Total Phenolic Content (TPC)
Phenolic compound are able to absorb free radicals and can chelate metal ions that could catalyze formation of ROS which promotes lipid peroxidation. Phenolic compounds have redox properties, acting as free radical scavenging or primary antioxidants; therefore, it is justifiable to determine phenolic content in plant extract. Total phenolic content of different fractions of M. koenigiiare shown in (Figure 1). Among the fractions, the highest phenolic content was found in Dia-ion resin adsorbed fraction (17.52 mg GAE/gm of extract), followed by Ethyl acetate fraction (14.27 mg gallic acid/gm of extract), Chloroform fraction (13.285 mg gallic acid/gm of extract) and petroleum ether fraction (13.25 mg gallic acid/gm of extract).
Figure 1.Total phenolic content (mg GAE/g extract ) of different extract of M. koenigii.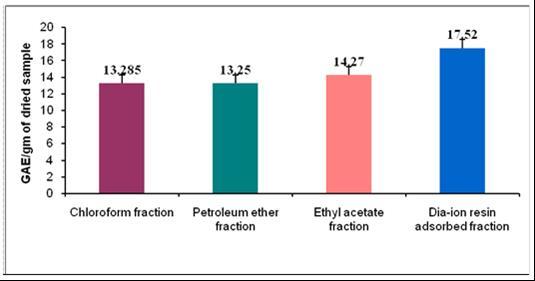
Total Flavonoid Content (TFC)
Plant flavonoids are the secondary metabolites with both in vitro and in vivo antioxidant activity and they help human body to defend against oxidative stress related diseases25. Total flavonoid content of different fractions of M. koenigiiare shown in (Figure 2). Among the fractions, the highest total flavonoid content was found in Dia-ion resin adsorbed fraction (16.65 mg CatE/g of extract), followed by Chloroform fraction (14.60 mg CatE/g of extract), Ethyl acetate fraction (13.42 mg CatE/g of extract) and petroleum ether fraction (12.83 mg CatE/g of extract). Therefore, it can be said that polyphenolic, and flavonoid may work together with other phytochemicals present in M. koenigii and make it medicinally important.
Figure 2.Total flavonoid content (mg CatE/g extract) of different fractions of M.koenigii.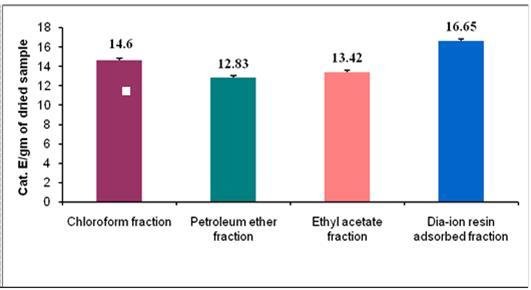
Reducing Power Capacity Content
The reducing potential of plant is mostly associated with the presence of reductones, which exert their mechanism of action by breaking the free radical chain by donating a hydrogen atom. The iron reducing capacity of the four different fractions of Murrayakoenigiiextract such as petroleum ether fraction (PE), chloroform fraction (CE), ethyl acetate (EE) and Dia-ion resin- adsorbed fraction (DAF) have been investigated. Among the four different extracts Dia-ion resin-adsorbed fraction showed the highest iron reducing capacity with absorbance of 2.504 at 80 mg/ml concentration, followed by Chloroform fraction with absorbance 2.263±0.003 at 80 mg/ml while Ethyl acetate fraction showed iron reducing capacity with absorbance of 1.675 at 80 mg/ml and Petroleum ether fraction showed the iron reducing capacity with absorbance 1.421 at 100 mg/ml (Figure 3). Different extracts, and standard (vitamin C, AA) exhibited the reducing power activity as follows: AA>DAF > CE> EE > PE.
Figure 3.Reducing power capacity of different fractions of methanolic extract of M.koenigii and Ascorbic acid (Standard).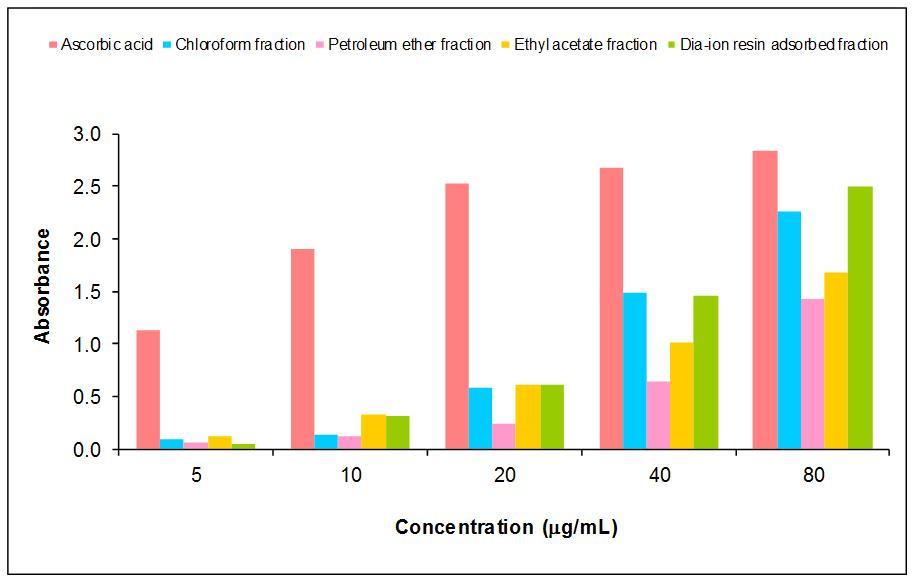
Total Antioxidant Activity
The antioxidant ability and radical scavenging properties of plants are associated with its medicinal values. Total antioxidant activity of different fractions of methanolic extract of Murrayakoenigii leaves such as Dia-ion resin adsorbed fraction, chloroform fraction, Ethyl acetate fraction and petroleum ether fraction were investigated. Among the fractions, Dia-ion resin adsorbed fraction showed the highest total antioxidant activity with absorbance 2.320 at 100 mg/ml (Figure 4), whereas the Chloroform fraction and Ethyl acetate fraction showed absorbance 2.306 at 100 mg/ml and 2.183 at 100 mg/ml respectively. Petroleum ether fraction showed the lowest total antioxidant activity with absorbance 1.944 at 100mg/ml concentration. Different extractives, and standard exhibited the antioxidant activity as follows: AA> DAF> CE> EE> PE
Figure 4.Total antioxidant activity of different fractions of methanolic extract of M.koenigii and Ascorbic acid (Standard).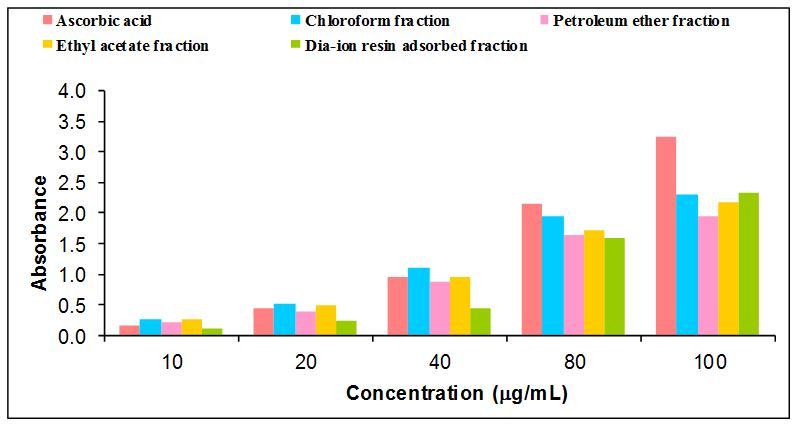
DPPH Free Radical Scavenging Activity
Plants rich in secondary metabolites, including phenolics and flavonoids, have antioxidant activity due to their redox properties and chemical structures. The DPPH radical is widely used in assessing free radical scavenging activity because of the ease of the reaction. Among the fractions of the extract, highest DPPH radical scavenging activity was found in Dia-ion resin adsorbed fraction having IC50 value 15.53mg/ml. On the other hand, chloroform fraction showed DPPH radical scavenging activity with IC50 value 24.62mg/ml, followed by ethyl acetate fraction with IC50 value 21.97mg/ml and petroleum ether fraction showed DPPH radical scavenging activity with IC50 value 18.56 mg/ml (Figure 5 and Figure 6).
Figure 5.DPPH radical scavenging activity of different fractions of methanolic extract of M. koenigii and BHT (standard).
Figure 6.IC50 values of different extractives of M.koenigii and standard BHT.
Conclusion
The present study investigated the antioxidant activity of different extracts of the plant M. koenigii. For this purpose, total phenolic content, total flavonoid content, reducing power capacity, total antioxidant, and DPPH radical scavenging activity tests were performed with four different fractions of the plant. From the above results, we can conclude that, Dia-ion resin-adsorbed fraction showed the highest activity in Total phenolic content, total flavonoid content, Reducing power capacity, Total antioxidant and DPPH radical scavenging. Dia-ion resin is good adsorbent for separatin polyphenolic compounds. Plant polyphenols are potent antioxidants widely distributed and accumulated in large amount in various plants consumed by human beings. Plants polyphenols scavenge DPPH free radicals. Total polyphenols were significantly negatively correlated to IC50 values of DPPH radicals scavenging. Plant flavonoids are health-promoting, disease-preventing dietary antioxidant compounds that have been shown in numerous in vitro and in vivo experiments. In the present study phenolic compound were found to be the predominant components in the leaves of M. koenigii indicating that they are potent antioxidant.
Acknowledgement
We are thankful to the authority of BCSIR Laboratories, Rajshahi and Rajshahi University for their technical support for carrying out this research work.
References
- 1.Manvi G P. (2010) Screening and evaluation of pharmacognostic, phytochemical and hepatoprotective activity. , of J. communis L. Stems. Int. J. Pharm. BioSci 1, 3.
- 2.Adetutu A, W A Morgan, Corcoran O. (2011) Ethnopharmacological survey and in vitro evaluation of wound-healing plants used on South-western Nigeria. , J. Ethnopharmacol 137, 50-56.
- 3.Gurung S, N S Basnet. (2009) Wound healing properties of Carica papaya latex: In vivo evaluation in mice burn model. , J. Ethanopharmacol 121, 338-341.
- 4.Suntar I, E K Akkol, Keles H, Oktem A, Baser K H C et al. (2011) A novel wound healing ointment: A formulation of Hypericum perforatum oil and sage and oregano essential oils based on traditional Turkish knowledge. , J. Ethanopharmacol 134, 89-96.
- 5.Balekar N, N G Katkam, Nakpheng T, Jehtae K, Srichana T. (2012) Evaluation of the wound healing potential of Wedelia trilobata (L.) leaves. , J. Ethanopharmacol 141, 817-824.
- 6.Kumar B, Viayakumar M, Govindarajan G, Pushpangadan P. (2007) Ethanopharmacological approaches to wound healing-Exploring medicinal plants of India. , J. Ethanopharmacol 114, 103-113.
- 7.Shailajan S, Menon S, Pednekar S, Singh A. (2011) Wound healing efficacy of Jatyadi Taila: In vivo evaluation in rat using excision wound model. , J. Ethanopharmacol 138, 99-104.
- 8. (2003) IUCN-The World Conservation Union, Bio-ecological Zones of Bangladesh. Bangladesh Country Office.
- 9.Ghani A. (2003) Medicinal plants of Bangladesh: Chemical constituents and uses. 2nd ed. , Dhaka, Asiatic Society of Bangladesh 309-10.
- 10.Rahman M M, Islam W, Ahmed K N. (2009) Functional response of the predator Xylocoris flavipes to three stored product insect pests. , Int. J. Agric. Biol 11, 316-320.
- 11.Swingle W T, P C Reece. (1967) The botany of Citrus and its wild relatives of the orange subfamily (family rutaceae Subfamily Aurantioideae), In: The Citrus Industry. , ed Reuther W et 1, 190-430.
- 12.V K Ranade, R K Lal, Tripathi S, Khan M, Syamasunder K V et al. (2002) Essential oil composition of genetically diverse stocks of Murraya koenigii from India. , J. Flavour Fragrance 17, 144-146.
- 13.Prajapati N D, Purohit S S, Sharma A K, Kumar T. (2003) . In; A andbook of Medicinal Plants, 1st,Edn., Agrobios India 401.
- 14.Rao B R R, D K Rajput. (2011) Mallavarapu GR. Chemical diversity in curry leaf (Murayya koenigii) essential oil, Food Chem. 126(3), 989-994.
- 15.Jyoti D, Anupam S, Ashwani K, Jitender S. (2016) Isolation, characterization and quantification of an anxiolytic constituent - mahanimbine, from Murraya koenigii Linn. Spreng Leaves. , Journal of Ethnopharmacology 193-706.
- 16.Adebajo A C, Avoola O F, Iwalewa E O, Akindahunsi A A, Omisore N O et al. (2006) Anti-trichomonal, biochemical and toxicological activities of methanolic extract and some carbazole alkaloids isolated from the leaves of Murraya koenigii growing in Nigeria. , Phytomedicine 13(4), 246-54.
- 17.Jain V, Momin M, Laddha K. (2012) Murraya Koenigii: An Updated Review”. , International Journal of Ayurvedic and Herbal Medicine 2(2), 607-627.
- 18.Sankar G, Ravishankar R V. (2015) In vitro antibiofilm activity of Murraya koenigii essential oil extracted using supercritical fluid. CO2 method against Pseudomonas aeruginosa PAO1. Natural Product Research 29(24), 2295-2298.
- 19.Kumpulainen J T, Salonen J T. (1999) Natural Antioxidants and Anticarcinogens. in Nutrition, Health and Disease, The Royal Society of Chemistry, UK 178-18.
- 20.Singleton V L, Rossi J A. (1965) Colorimetry of total phenolics with phosphomolybdic- phosphotungstic acid reagents. , American Journal of Enology and Viticulture 16, 144-158.
- 21.Chang C, Yang M, Wen H, Chern J. (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. , J. Food Drug Anal 10, 178-182.
- 22.Stankovic M S. (2011) Total phenolic content, flavonoid concentration and antioxidant activity of Marrubium peregrinum L. extracts. , Kragujevac J. Sci 33, 63-72.
- 23.Prieto P, Pineda M, Aguilar M. (1999) spectrophotometic quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. , Anal. Biocbem 269, 337-341.
Cited by (1)
- 1.Sandamali Jayasinghe A. N., Hewawasam Ruwani P., Jayatilaka Kamani A. P. W., Mudduwa Lakmini K. B., Rios José L., 2020, Cardioprotective Potential of Murraya koenigii (L.) Spreng. Leaf Extract against Doxorubicin‐Induced Cardiotoxicity in Rats, Evidence-Based Complementary and Alternative Medicine, 2020(1), 10.1155/2020/6023737
