Author Contributions
Academic Editor: Biyu Shen, Department of Nursing, The Second Affiliated Hospital of Nantong University
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2016 Vikas Dhikav, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Alteration of the hypothalamic-pituitary-adrenal (HPA) axis has been observed in patients with Alzheimer’s disease (AD) 1-5. Changes in Cortisol levels in patients with AD prompted the hypothesis that stress and glucocorticoids are involved in the development or maintenance of AD6. Glucocorticoids might also influence amyloid beta levels and their deposition in patients with AD6. A small study (n=9) conducted almost a decade ago demonstrated that serum cortisol is mildly elevated in AD and could be related to disease progression7, 8. The association between apolipoprotein E Epsilon 4 (APOE ɛ4) and serum cortisol also suggests that the two common pathophysiological links i.e. amyloid beta and APOE ɛ4 areinterrelated9. Prior studies have demonstrated that Medial Temporal Lobe (MTL) could be affected by AD/MCI. The importance of Medial Temporal Lobe Atrophy (MTLA) with respect to cognitive decline is further established by hippocampus’s role in memory formation10, 11
Chronically elevated cortisol levels have been associated with increased blood pressure and cerebral atrophy 10but its relationship with cognition seems to be a complex one. In one study of older adults (n=27), significant correlation between the HPA axis hyperactivity and frontal lobe atrophy10was observed.
MTLA is more common in patients with AD/MCI with comorbid depression compared to those without2. There is an elevation of serum cortisol in some patients with AD and in major depression as well9. Hippocampal area, the major component of the medial temporal lobe is sensitive to the toxic effects of glucocorticoids and undergoes atrophy under the influence of its chronic elevation. Therefore, the relationship between MTLA, serum cortisol and depression seems plausible. In the present study, an attempt was made to see if a correlation exists between basal Serum Cortisol, Depression and Medial Temporal Lobe Atrophy Visual Rating Scores in patients with AD/MCI.
Material and Methods
Screening
We screened 60 patients with complaints of subjective memory impairment presenting to the Department of Neurology at a tertiary care centre starting from July 2012 to July 2015 and enrolled 28 of them into the study. These patients met the diagnostic Criteria for diagnosis of Dementia of Probable Alzheimer’s disease ((National Institute of Neurological and Communicative Disorders and Stroke, Alzheimer’s disease related Disease Association criteria (NINCDS-ARDA)). For the diagnosis of MCI, Clinical Dementia Rating Scale (CDR Score=0.5) was used. Dementias other than AD were excluded from the study.
Patient Selection and Diagnostic Evaluation
Selected patients underwent detailed neuropsychological, radiological and neurological examination for diagnosis of AD. A detailed general physical examination was conducted in all cases to rule out systemic diseases that could have accounted for the cognitive impairment besides AD. Routine laboratory examination and work up to rule out other types of dementias was also done.
A written and informed consent was obtained from all study participants. The study was approved by institutional ethics committee.
Study Design
This is a cross sectional and observational study in which recruited patients with AD/MCI were subjected to Magnetic Resonance Imaging of the Brain and serum cortisol measurement. Serum cortisol was then correlated to MTLA and depression. Selected patients were free from concurrent neurological or mental disorders. All were but 3 were past non-smokers and non-alcoholics.Serum cortisol levels for patients with depression were assessed before starting them on antidepressants or any other treatments/s for dementias (e.g. anticholinesterases). Later on, patients with depression were treated with antidepressants (e.g. ecitalopram/sertraline) or with anticholinesterases (e.g. donepezil/rivastigmine).
Scales
Mini Mental Status Examination (MMSE): to cognitive screen and categorise patients into mild, moderate and severe cognitive impairment. MMSE is a tool that is used to systematically and thoroughly assess mental status. It is an 11-question measure that tests five areas of cognitive function: orientation, registration, attention and calculation, recall, and language.
Scheltens Visual Rating of Medial Temporal Lobe Atrophy: The visual rating of MTLA was done using this scale5by obtaining T1 weighted coronal section image on MRI scan of the brain. This rating does not require any special radiological training and can be done easily using hard copies of T1 weighted coronal sections of the brain
MRI. It has a diagnostic accuracy of over 80% in diagnosing dementia of Alzheimer’s type (Table 1).
Table 1. Methodology of Scheltens Visual Rating for Medial Temporal Rating Scale| Scoring | Interpretation |
| 0 | No atrophy |
| 1 | Minimal atrophy |
| 2 | Mild atrophy |
| 3 | Moderate atrophy |
| 4 | Severe atrophy |
Cornell Scale for Depression in Dementia (CSDD): considered to be the Gold Standard for assessment of depression in Dementia. This scale is the best validated instrument for assessing depression in dementia and takes into account that some of the symptoms of dementia can mimic those of depression. CSDD involves a comprehensive semi-structured interview to reach a composite clinical rating. Depression was evaluated using CSDD. Those with AD/MCI and CSDD scores greater than 10 were included for cortisol level estimation (10 is the cut off score for depression in dementia).
Clinical Dementia Rating Scale: The Clinical Dementia Rating is a five-point scale in which CDR-0 connotes no cognitive impairment, and then the remaining four points are for various stages of dementia: The CDR 0.5 is considered to be mild cognitive impairment.
All the scales used in the current study have been well validated and extensively used in Indian patients2, 22, 23, 24.
Radiology Protocol
MTLA was assessed using a template based on Scheltens Visual Rating Scale (Figure 1). MRI scans were done using 1.5 Tesla Magnetic Resonance (Megnatom Symphony 1.5T scanner). T1 weighted Coronal sections were used for rating MTLA.
Figure 1.Scheltens Visual Rating Scale used in the current study to assess the medial temporal lobe atrophy rating scores.
Biochemical Analysis
After an overnight fasting, morning samples (8 AM) of 5 ml whole blood were withdrawn from the antecubital vein. A total of 3 ml serum was extracted and estimation was done using Chemiluminescence based competitive assay. A reference range of 10-20 microgram/dl was used as normal. This study was approved by the Institutional Ethical Committee.
Statistical Analysis
The latest version of Statistical Package for Social Sciences (SPSS®-SPSS Inc., Chicago, IL) was used for data analysis. Normality of data was checked using Q-Q plot. Correlations and Pearson Correlation Coefficients were assessed for various variables. Linear regression was done. Differences between left and right medial temporal lobe ratings were compared using paired t-test. Two tailed p- value <0.05 was used to test the level of significance. Confidence interval (95%) was calculating using value of Pearson Correlation Coefficient (r).
Results
A total of 28 Patients (M=24: F=4) presenting with subjective memory complaints/cognitive abnormalities to the Department of Neurology of a tertiary care hospital in India were assessed. These included 15 patients with MCI and 13 with AD. The mean age was 73.39 ±7.6 years and mean duration of illness was 3.4±3 years. The mean MMSE Score was 21.7±7.4. Mean CSDD score was 11.6±6 and mean MTLA scores of both sides were 1.5±0.74.
There was no statistical difference between left and right MTLA scores (p=>0.05) using paired t test. Mean basal serum cortisol level was 15.3±6.3 microgram/dl for all patients and 18±7 microgram/dl for patients with AD. Four patients (14%) had a high morning serum cortisol. Only one case out of those 4 was MCI, rest three had AD (mean MMSE=15.3±3).
There was a significantly positive correlation (Figure 2) between serum cortisol level and MTLA scores (Pearson Correlation Coefficient=0.39, p<0.05). Similarly, a significantly positive correlation was present between serum cortisol and CSDD Scores (Pearson Correlation Coefficient=0.49, p<0.05).
Figure 2.Linear regression showing correlation between serum cortisol (y) and MTA scores (x).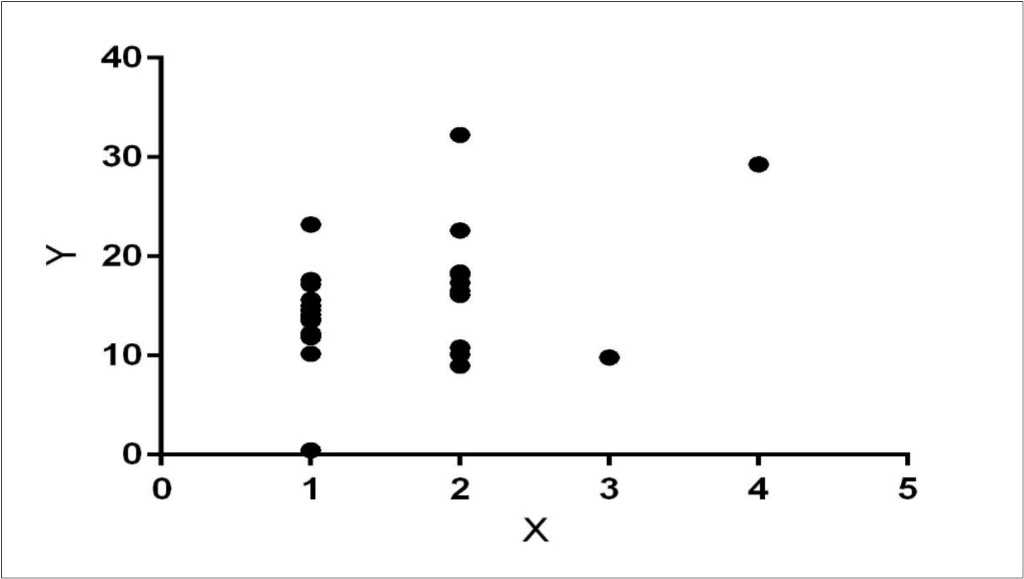
There was a significantly negative correlation between MMSE and MTA scores (Pearson Correlation Coefficient=0.60, p<0.0001). No significant correlation between age and MTA scores was observed. A positive correlation between CSDD and MMSE was also present (r=0.40, p<0.05). One way Analysis of Variances (ANOVA) test was performed using standard weighted-means analysis to see if the groups with MMSE scores, MTA scores, serum cortisol and CSDD scores were significantly different from each other. ANOVA showed a highly significant difference between groups (p<0.0001). Tukey’s Honestly Significant Difference Test showed that all 4 independent groups at alpha level of significance (0.05) were significantly different from each other. Odds ratio of serum cortisol in AD/MCI was 3.4 (95% confidence interval=0.3197 to 37.4751), however the p-value was not significant.
Discussion
Alteration in serum cortisol has been observed in prior studies involving AD patients1. This cortisol elevation could possibly happen as a response to stress. Dysregulated hypothalamic-adrenal-pituitary axiscan cause damage to the Hippocampus, a structure located in medial temporal lobe, important for learning and memory. Poor memory and mis-communication2may propound stress. A greater severity of dementia is associated with rise in serum cortisol3. In our study as well, high serum cortisol was found in 14% of the patients with AD/MCI. Earlier studies have demonstrated that higher/rising serum cortisol is associated with rapid disease progression4. It has been postulated that the negative feedback of the shrunken hippocampus becomes weak and is unable to exert its inhibitory effect and hence leads to hypercortisolemia2.
Qualitative visual assessment of Temporal Lobe Atrophy on MRI has high sensitivity in distinguishing AD from normal aging, depression, and vascular dementia2, 9, 10. Atrophy of the MTL is particularly important because it can be detected earlier than generalized atrophy in patients with AD2, 11, 12, 13. No prior studies have correlated serum cortisol levels with MTLA scores, although they have been correlated with hippocampal volumes in the past14. We wanted to see if the same correlation can be observed using a visual rating scale as well. This is important as MTLA can be assessed quickly using the visual rating as a bedside tool in busy clinics. Mild elevation of serum cortisol has been observed in patients with ADin the previous studies1. We observed 4 patients with cortisol elevation in this series. Since we had more number of patients with MCI than AD; therefore frank hyper-cortisolemia was not seen. Moreover, our patients had moderate AD (mean MMSE=15.3±3) rather than severe AD which are more likely to have higher cortisol levels3, 4, 5. In general MCI patients do not have very high serum cortisol levels. In fact patients with MCI might have even lower than normal adult levels of serum cortisol which makes the association between cortisol and AD/MCI a complex one15. Some of the findings of this study are similar to our earlier study, where we demonstrated that MTLA is more common in patients with AD/MCI with depression compared to those without2. In this study, there was a negative correlation between MMSE and CSDD suggesting that patients with decreased cognition (severe dementias) are more likely to have depression.
A silico-model of hippocampal dysfunction correlated hippocampal activity (HA) and serum cortisol. This model predicted that aging induces a 12% decrease in HA. Acute and chronic elevations in cortisol decreased the HA further to 30% and 40% respectively. A biological intervention used in the study attenuated the cortisol induced decrease in HA by 2% in the acute cortisol simulation and by 8% in the chronic simulation16. This suggests that higher serum cortisol levels may potentially have a negative consequence for hippocampus and for disease in general. How much of this is applicable to AD/MCI currently remains unknown16.
In a recent study of 155 patients, serum cortisol was found to have distinct ability to differentiate AD patients from healthy controls17. However, the contributory effect of serum cortisol towards cognitive decline remains uncertain18, 19, 20, 21. In the present study, cortisol levels of patients with AD were higher than the mean of all patients (AD+MCI) combined together (18±7 microgram/dl) and yet the levels were within normal range. Our results are in agreement with the Rotterdam study where serum cortisol of larger number of patients was studied (n=90)19. Our study also confirms the findings of a study2 that observed correlations between MTLA and depression. In addition, we have shown correlation between serum cortisol and MTLA. Correlation between serum cortisol and MTA seems in accordance with animal data and the proposed hypothesis that higher cortisol might contribute to MTLA3, 4, 5. However, larger study with greater sample size could be done to confirm these findings.
Present study also highlights an important issue related to depression in Dementia. The findings are in accordance with those of a previous study in patients with AD having comorbid depression2and further, this correlation can be confirmed using biochemical means as well. A negative correlation between MSSE and CSDD scores is also an interesting finding which points towards the fact that decreased cognition (as observed in Dementia), increases the possibility of depression. Conversely, a higher level of depression might contribute to decreased cognition. The study also establishes a strong negative correlation between MMSE and MTA scores (Pearson Correlation Coefficient=0.60, p<0.0001). This confirms that an increasing severity of dementia is associated with greater MTLA. A sex bias22, 23, 24 can be seen in the present study and can limit the external validity of the study apart from the small sample size. This sex bias is typical of memory disorders in several developing countries2 where females are hardly brought in for treatment.
The issue of depression in AD/MCI and MTA is getting increasing attention. For example, a recent large study (n=366) showed that hippocampal atrophy was more pronounced among patients having MCI with depressive symptoms. These findings suggest that different mechanisms underlie depression in older people with and without AD and may explain some of the inconsistent observations in previous studies25. Additionally, the Leukoaraiosis and Disability in the elderly (LADIS) study26 which was another large study (n=639) that showed that the depressive symptoms are associated with an increase risk of cognitive decline, independent of the effect of white matter lesions in brain, probably due to an additive or synergistic effect. In this context, depressive symptoms probably represent a subtle ongoing organic dysfunction. A small pilot study also suggested that amnestic MCI (aMCI) can be distinguished from late life depression based on cerebral atrophy measures and that the hippocampal and entorhinal atrophy in aMCI varies according to the presence or absence of depressive symptoms27. The latter suggests that not only can MTLA be used to distinguish different types of dementias, but it is also valuable as a bedside tool to differentiate aMCI from late life depression.
Conclusion
A statistically significant correlation between serum Cortisol and MTA Scores as well as between MTA Scores and CSDD scores highlights the importance of glucocorticoids as a potential contributor towards Medial Temporal lobe Atrophy in patients with AD/MCI.
Acknowledgements
The authors wish to thank the entire staff of Guru Gobind Singh Indraprastha University, New Delhi, INDIA for their support. Also, the help of Mansi Sethi, Research Volunteer, Department of Behavioral Health Henry ford hospitals, Detroit, Michigan USA and Ms Dolly Prajapati of Department of Neurology, Dr. RML Hospital & PGIMER, New Delhi, INDIA for helping to improve the quality of manuscript before final publication.
References
- 1.Dhikav V, Anand K S. (2007) Glucocorticoids may initiate Alzheimer's disease: a potential therapeutic role for mifepristone (RU-486). Med Hypotheses. 68(5), 1088-92.
- 2.Dhikav V, Sethi M, Anand K S. (2014) Medial temporal lobe atrophy in Alzheimer's disease/mild cognitive impairment with depression. , Br J Radiol 87(1042), 20140150.
- 3.Giubilei F, Patacchioli F R, Antonini G, Sepe Monti M, Tisei P et al.Altered circadian cortisol secretion in Alzheimer's disease: clinical and neuroradiological aspects. , J Neurosci Res.2001oct15; 66(2), 262-5.
- 4.Csernansky J G, Dong H, Fagan A M, Wang L, Xiong C et al. (2006) Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. , Am J Psychiatry 163(12), 2164-9.
- 5.Notarianni E. (2013) Hypercortisolemia and glucocorticoid receptor-signaling insufficiency in Alzheimer's disease initiation and development. Curr Alzheimer Res. 10(7), 714-31.
- 6.A1 Brureau, Zussy C, Delair B, Ogier C, Ixart G et al. (2013) Deregulation of hypothalamic-pituitary-adrenal axis functions in an Alzheimer's disease rat model. Neurobiol Aging. 34(5), 1426-39.
- 7.MF1 Weiner, Vobach S, Olsson K, Svetlik D, Risser R C. (1997) Cortisol secretion and Alzheimer's disease progression. Biol Psychiatry. 42(11), 1030-8.
- 8.A1 Singh-Manoux. (2014) Dugravot A2, Elbaz A2, Shipley M3, Kivimaki M3, Kumari M3. No evidence of a longitudinal association between diurnal cortisol patterns and cognition. Neurobiol Aging. 35(10), 2239-45.
- 9.BK1 Lee, Glass T A, Wand G S, McAtee M J, Bandeen-Roche K et al. (2008) Apolipoprotein e genotype, cortisol, and cognitive function in community-dwelling older adults. , Am 165(11), 1456-64.
- 10.SM1 Gold, Dziobek I, Rogers K, Bayoumy A, McHugh P F et al. (2005) Hypertension and hypothalamo-pituitary-adrenal axis hyperactivity affect frontal lobe integrity. J Clin Endocrinol Metab. 90(6), 3262-7.
- 11.BG1 Rathakrishnan.Doraiswamy PM2 and Petrella JR3. Science to practice: translating automated brain MRI volumetry in Alzheimer's disease from research to routine diagnostic use in the work-up of dementia. , Front Neurol.2014Jan9 4, 216.
- 12.O’Brien J T, Desmond P, Ames D, Schweitzer I, Chiu E et al. (1997) Temporal lobe magnetic resonance imaging can differentiate Alzheimer’s disease from normal aging, depression, vascular dementia, and other causes of cognitive impairment. , Psychol Med 27(6), 1267-7510.
- 13.Burton E J, Barber R, Mukaetova-Ladinska E B, Robson J, Perry R H et al. (2009) Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain. 132(1), 195-20310.
- 14.JC1 Pruessner, Baldwin M W, Dedovic K, Renwick R, Mahani N K et al. (2005) Self-esteem, locus of control, hippocampal volume and cortisol regulation in young and old adulthood. , Neuroimage 28(4), 815-26.
- 15.M1 Pruessner, Pruessner J C, Hellhammer D H, Bruce Pike G, Lupien S J. (2007) The associations among hippocampal volume, cortisol reactivity, and memory performance in healthy young men. Psychiatry Res. 155(1), 1-10.
- 16.MT1 McAuley, Kenny R A, Kirkwood T B, Wilkinson D J, Jones J J et al. (2009) A mathematical model of aging-related and cortisol induced hippocampal dysfunction. , BMC Neurosci 10, 26.
- 17.C1 Laske, Leyhe T, Stransky E, Hoffmann N, Fallgatter A J et al. (2011) Identification of a blood-based biomarker panel for classification of Alzheimer’s disease. , Int J Neuropsychopharmacol 14(9), 1147-55.
- 18.CR1 Kovach, Woods D L, Logan B R, Raff H. (2011) Diurnal variatin of cortisol in people with dementia: relationship to cognition and illness burden. Am J Alzheimers Dis Other Demen. 26(2), 145-50.
- 19.EM1 Schrijvers, Direk N, Koudstaal P J, Kirschbaum C, Hofman A et al. (2011) Associations of serum cortisol with cognitive function and dementia: the Rotterdam Study. J Alzheimers Dis. 25(4), 671-7.
- 20.Wolf O T, Convit A, Thorn E, de Leon MJ. (2002) Salivary cortisol day profiles in elderly with mild cognitive impairment. Psychoneuroendocrinology. 27(7), 777-89.
- 21.Singh-Manoux A, Dugravot A, Elbaz A, Shipley M, Kivimaki M et al. (2014) No evidence of a longitudinal association between diurnal cortisol patterns and cognition. Neurobiol Aging. 35(10), 2239-45.
- 22.Dhikav V, Sethi M, Mishra P, Singh Anand K. (2015) Behavioral and psychological symptoms among Indian patients with mild cognitive impairment. Int Psychogeriatr. 29, 1-2.
- 23.Dhikav V, Singh P, Anand K S. (2013) Medication adherence survey of drugs useful in prevention of dementia of Alzheimer's type among Indian patients. , Int Psychogeriatr 25(9), 1409-13.
- 24.Dhikav V, Anand K S. (2012) Caregiver burden of behavioral and psychological symptoms of dementia among Indian patients with Alzheimer’s disease. , Int Psychogeriatr 24(9), 1531-2.
- 25.Enache D, Cavallin L, Lindberg O, Farahmand B, Kramberger M G et al. (2015) Medial temporal lobe atrophy and depressive symptoms in elderly patients with and withoutAlzheimer disease. , J Geriatr Psychiatry Neurol 28(1), 40-8.
Cited by (1)
- 1.Keihani Ahmadreza, Mayeli Ahmad, Ferrarelli Fabio, 2023, Circadian Rhythm Changes in Healthy Aging and Mild Cognitive Impairment, Advanced Biology, 7(11), 10.1002/adbi.202200237
