Abstract
Spontaneous intracerebral hemorrhage (ICH) is one of the leading causes of death worldwide. In randomized trials on surgical therapy inclusion of the very old was limited by the recruitment process. This study was performed to evaluate the age limits in published surgical trials on ICH, and to determine how upper age limits effect the inclusion of men and women in these and future trials on the basis of a large cohort of ICH patients in central Europe.
The Hessian stroke registry, a state-wide prospective stroke databank, was used to analyze upper age limits and sex differences for patients with the diagnosis of ICH (ICD-10: I61.0 to I61.9) who were admitted between January 2010 and December 2012. Sex differences were calculated at different age cutoffs, and the proportions of potentially excluded sex-specific patients from surgical trials on ICH were calculated.
Overall, 5184 patients with the diagnosis of spontaneous ICH were identified. A total of 2457 (47.4%) patients were female and 2727 (52.6%) patients were male. Mean age was 72.3 ± 13.6 years. Female patients were significantly older compared to male patients (74.9 ± 13.5 years vs. 69.9 ± 13.2 years; p<0.001). With an upper age limit of 70, 75, and 80 years, 3437 patients (66.3%), 2664 patients (51.4%), and 1765 patients (34.0%) were excluded, respectively.
Upper age limits in surgical trials on ICH could lead to the exclusion of a significant portion of patients from studies. This should be noted when transferring conclusions from these trials into clinical practice.
Author Contributions
Academic Editor: Biswarup Ghosh, Thomas Jefferson University, Dept. of Neuroscience, Philadelphia, US
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2016 Marco Stein, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Spontaneous intracerebral hemorrhage (ICH) accounts for 11-22% of all strokes worldwide.1 The early case fatality rate was 25–35% in the first month. Incidence of ICH increases with advanced age.2 Over the coming decades, the aging of the population will force a major shift in clinical care of patients with ICH, and the group of patients older than 80 years will increase by the factor 2.5 from 2009 to 2050 in middle Europe.3 Differences of life expectancy between males and females lead to a higher proportion of females in the very old. This results in a higher rate of females in older patients with ICH.
In stroke trials, upper age limits were used to exclude patients from these studies for different reasons.4 For older individuals, higher rates of in-hospital mortality and higher rates of moderate or severe neurological deficits were noted.5 However, the aging of the population worldwide will create a serious challenge in the treatment of ICH in the coming decades. If current treatment concepts in the very old continue and incidence rates of ICH remain stable in this patient group, the demographical changes will lead to higher in-hospital mortality rates and higher rates of patients with severe disabilities.
Since the first published randomized surgical trial on ICH by McKissock et al.6 in 1961, several randomized surgical trials on ICH have been published. To date, no randomized surgical trial on open hematoma evacuation by craniotomy after ICH has been able to show that this procedure is superior to conservative treatment. Some randomized trials that include minimally invasive surgical concept at least in one arm of the study show a benefit of these procedures in special circumstances7, or a slightly improved functional outcome.8, 9
Our study was conducted to evaluate the upper age limits in published randomized surgical trials on ICH, and to calculate the proportions of potentially excluded patients for different upper age limits in a large representative cohort of patients with spontaneous ICH in central Europe.
Methods
We performed a systematic literature search on randomized controlled trials (RCT) on surgical interventions in patients with ICH in Medline and Pubmed. Only RCTs with at least one surgical arm were included. Only RCTs published in English language were included. Published RCTs in other languages were not included in this analysis. This literature search was performed to determine the age limitations of the study populations in published RCTs on ICH.
The data used for the evaluation of upper age limits were obtained from a state-wide prospective stroke registry in the state of Hesse, Germany, between January 2010 and December 2012. This stroke registry is mandatory for all patients with ischemic stroke, ICH, and subarachnoid hemorrhage that were admitted to an acute care hospital. In comparison to billing records from health insurance data a documentation rate of 95.5% was achieved in the Hessian stroke registry.10 In this study only patients with the diagnosis of first-ever or recurrent ICH were included. The clinical parameters included in this analysis were prospectively documented.
Basis of the stroke registry in Hesse are Data from a quality assurance program which is regulated by the German Social Code, Book Five (SGB V). Patients’ data were registered anonymously; therefore no ethical board approval was necessary. Early outcome was determined with the modified Rankin Scale (mRS). Pre-ICH disability was defined as mRS>2 prior to ictus.
Statistical Analysis
Means or medians of age, and standard deviations or interquartile range (if applicable) were calculated from the published manuscripts and were rounded to one decimal figure.
We calculate the proportions of men and woman who will not be included in randomized surgical trials on ICH when different age limits are set (70, 75, and 80 years).
Data were analysed using the Student t test or the Mann-Whitney Utest for the comparison of continuous variables, and the chi-square test for the comparison of categorical variables.
Results:
Randomized Surgical Trials on ICH
Seventeen published randomized surgical trials on ICH with a total of 3472 patients met the inclusion criteria of this study (Table 1). In 11 (68.8%) trials an age limit was part of the inclusion criteria. Mean or median age was reported in 15 (88.2%) trials. Mean age ranges from 51.7 years11 to 68.0 years12. An upper age range of included patients was noted in 11 (64.7%) trials and ranges from 65 years11 to 94 years.13 An upper age limit was part of the inclusion criteria of 10 (58.8%) trials. Sex ratio was documented in 16 trials. In total, 1399 (40.5%) female and 2052 (59.5%) male patients were recorded.
Table 1. Age and gender distributions in published randomized surgical trials on intracerebral hemorrhage.| Author | Year | Age (Years * ) | Age, (Range) | Age limit (Inclusion criteria) | Sex (Female/Male) | Patients (N =) |
| McKissock et al.6 | 1961 | n/a | 32-76 | None | 90/90 | 180 |
| Auer et al.7 | 1989 | n/a | n/a | 30-80 | 39/61 | 100 |
| Juvela et al.11 | 1989 | 51.7±9.5 | 15-65 | None | 22/30 | 52 |
| Batjer et al.17 | 1990 | 54.0±3.9 | n/a | 30-75 | n/a | 21 |
| Morgenstern et al.14 | 1998 | 53.5 (43-63)# | 22-77 | None | 12/22 | 34 |
| Zucarello et al.15 | 1999 | 62.4±11.9 | 27-80 | ≥18 | 9/11 | 20 |
| Tan et al.18 | 2001 | 56.3±9.0 | 38-70 | 35-70 | 6/28 | 34 |
| Teernstra et al.12 | 2003 | 68.0 | 46-87 | ≥45 | 30/40 | 70 |
| Mendelow et al.16 | 2005 | 62(52-70)# | 19-93 | None | 442/591 | 1033 |
| Hattori et al.20 | 2006 | 60.5±9.2 | n/a | 35-85 | 94/148 | 242 |
| Pantazis et al.19 | 2006 | 61.4±15.6 | 31-80 | >80 | 48/60 | 108 |
| Cho et al.21 | 2006 | 56.6±8.8 | 30-70 | 30-70 | 19/41 | 60 |
| Wang et al.8 | 2009 | 56.7±9.5 | 40-75 | 40-75 | 141/236 | 377 |
| Sun et al.9 | 2010 | 56.1±8.9 | n/a | 40-75 | 108/196 | 304 |
| Zhou et al.22 | 2011 | 58.2±8.8 | n/a | 40-75 | 43/79 | 122 |
| Mendelow et al.13 | 2013 | 63.9±13.3 | 17-94 | None | 257/340 | 597 |
| Mould et al.23 | 2013 | 60.7±11.8 | n/a | 18-80 | 39/79 | 118 |
Data from the Acute Stroke Care Project, State of Hesse, Germany
Between January 2010 and December 2012, 5184 patients with the diagnosis of spontaneous ICH (ICD-10: I61) were admitted to an acute care hospital in the state of Hesse, Germany. Main characteristics of the cohort are shown in Table 2. The median age in this cohort was 75 years with an interquartile range of 65 (P25) to 82 (P75), and ranges from 19 to 102 years. Except for diabetes, significantly higher rates of comorbidities were documented in patients with an age ≥80 years compared to patients less than 80 years. Of the 5184 patients, 2457 patients (47.4%) were female. Female patients were significantly older compared to male patients (74.9±13.5 vs. 69.9±13.2 years; p < 0.001) (Figure 1). Operative procedures (hematoma evacuation or EVD) were performed in 805 patients (15.5%). In the observed cohort, 1765 patients (34.0%) had an age ≥ 80 years and 3419 patients (66.0%) had an age >80 years. In the very old (age ≥80 years) the rate of operative procedures was significantly lower compared to patients <80 years [109 patients (6.2%) vs. 696 patients (20.4%); P <0.001]. Pre-ICH disability was documented in 827 patients (16.0%). In patients ≥80 years, the pre-ICH disability rate was significantly higher than in younger patients [432 patients (24.5%) vs. 395 patients (11.6%)]. In-hospital mortality was observed in 1247 patients (24.1%), and was significantly increased in patients ≥80 years compared to patients >80 years [620 patients (35.1%) vs. 627 patients 18.3%); P<0.001].
Table 2. Main characteristics of 5184 patients with spontaneous intracerebral hemorrhage by age.| N (%) | Overall (N = 5184) | <80 years (N =3419) | ≥80 years (N = 1765) | P-Value |
|---|---|---|---|---|
| Female | 2457 (47.4) | 1371 (40.1) | 1086 (61.5) | <0.001 |
| Age* | 72.3±13.6 | 65.6±11.9 | 85.2±4.1 | <0.001 |
| Pre-ICH disability | 827 (16.0) | 395 (11.6) | 432 (24.5) | <0.001 |
| Arterial hypertension | 3808 (73.5) | 2432 (71.1) | 1376 (78.0) | <0.001 |
| Diabetes mellitus | 813 (15.7) | 527 (15.4) | 286 (16.2) | 0.459 |
| Hypercholesterinaemia | 631 (12.2) | 393 (11.5) | 238 (13.5) | 0.038 |
| Atrial fibrillation | 958 (18.5) | 479 ( 14.0) | 479 (27.1) | <0.001 |
| GCS at admission# | 13 (7–15) | 13 (7–15) | 12 (7–15) | <0.001 |
| EVD | 588 (11.3 ) | 510 (14.9) | 44 (2.5) | <0.001 |
| Hematoma evacuation | 366 (7.1) | 322 (9.4) | 30 (1.7) | <0.001 |
| In-hospital Mortality | 1247 (24.1) | 627 (18.3) | 620 (35.1) | <0.001 |
Figure 1.Age distribution of 5184 patients with spontaneous intracerebral hemorrhage who were admitted to an acute care hospital in the State of Hesse, Germany, between January 2010 and December 2012. With an age of ≥80 years a significant increase in the proportion of female patients compared with an age <80 years exists (61.5% versus 40.1%; P<0.001).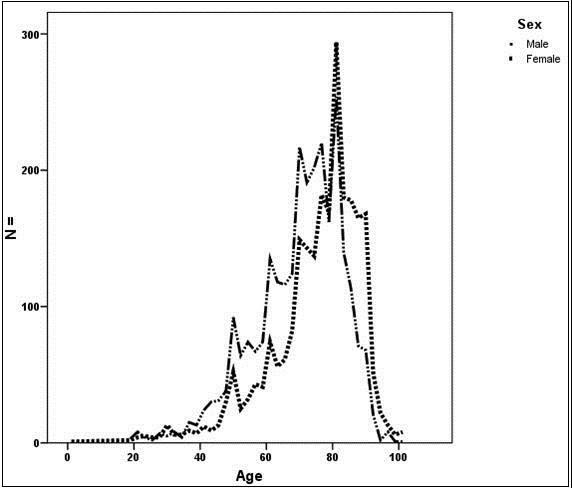
With an upper age limit of 70, 75, 80, 85, and 90 years in intracerebral hemorrhage trials, 3437 patients (66.3%), 2664 (51.4%), 1765 (34.0%), 897 (17.3%), and 259 (5.0%) will be excluded, respectively (Figure 2). In the very old, the cohort of patients with ICH was not gender neutral. With an age limit of 80 years, 1086 female patients (44.2% of all female patients), but only 679 male patients (24.9% of all male patients), were excluded (P<0.001).
Figure 2.Percentage of 5184 patients with spontaneous intracerebral hemorrhages that were potentially excluded from randomized surgical trials on intracerebral hemorrhage by different age limits. With an upper age limit of 70, 75, 80, 85, and 90 years 66.3%, 51.4%, 34.0%, 17.3%, and 5.0% will be excluded, respectively.
Discussions:
Summary of Findings
Upper age limits in RCT on surgical therapy of ICH leads to an exclusion of a considerable number of patients with ICH. The majority of published surgical trials on ICH defined age limitations in the inclusion criteria. Despite that no upper age limit was part of the inclusion criteria in 7 of the 17 evaluated RCT,6, 11, 16 only a few patients above the age of 80 were included in these trials.
There are two main findings of our study. First, with common upper age limits of 70, 75, and 80 years, a large group of ICH patients is excluded from participating in surgical RCT on ICH. Second, a significant gender mismatch exists in the very old with spontaneous ICH.
Concerning the first point: the goal of RCT on surgical intervention in ICH is to evaluate a therapy on patients who are representative for the population that is treated at a later time point. In this context, our analysis on mean or median age of published surgical trials on ICH shows that our study population was considerably older compared to published surgical trials on ICH. However, the presented data provides a representative overview on patients with spontaneous ICH outside of surgical RCT on this topic.
Concerning the second point: a significant gender mismatch exists in the very old with spontaneous ICH. With an age of 80 years or older the proportion of female patients is significantly higher through the differences in life expectancy between males and females (Table 2). The only published surgical trial on ICH with a balanced gender distribution is also the oldest trial.6 In all other included trials a strong domination of male patients in the study population exists (Table 1). When compared to our data, it appears that surgical therapies for ICH were evaluated with a disproportionately higher proportion of male patients; but this does not reflect the sex distribution in the very old.
Several surgical RCTs on open hematoma evacuation by craniotomy failed to show a clear benefit of this treatment.6, 11, 13, 14, 15, 16, 17, 18, 19 In our study, 9 of 17 trials evaluated the impact of open hematoma evacuation by craniotomy versus conservative therapy. However, 8 trials out of 17 had at least one minimally invasive arm. The prior experience of most clinicians suggested that very old individuals with spontaneous ICH will not tolerate an open hematoma evacuation operative procedure well. One theory for this hypothesis is that the procedure related injury to the normal brain and the length of anesthesia in the very old could mask the effect of the hematoma evacuation. However, an increase of RCTs on minimally invasive surgery can be observed in recent decades. Some positive effects were observed in these studies. Auer et al.7 found improved mortality and outcome rates in patients <60 years having smaller subcortical hematomas. In other studies, higher rates of independent survival after 90 days,8 or a decrease in the cumulative fatality rate at 90 days compared to conservative treatment,9 were observed. However, with the exception of the trial by Teernstra et al.,12 the very old were not represented in these trials.
Upper age limits in surgical RCTs are also part of the inclusion criteria of current trials on decompressive craniectomy after spontaneous ICH. In the current trial on decompressive hemicraniectomy in intracerebral hemorrhage SWITCH (NCT02258919), and in the trial on decompressive craniectomy after removing of intracerebral hematoma [CARICH (NCT02135783)], an upper age limit of 75 years and 80 years was part of the inclusion criteria. These upper age limits would have excluded over 50% of patients from the SWITCH trial, and over one third from the CARICH trial.
Our results should not be read as a request to open all future surgical RCT on ICH to very old individuals. The very old are special subgroup of patients and surgical or minimal invasive therapies for intracerebral hematomas should be evaluated in studies exclusively developed for this subgroup. In this context, the question is not only which method of surgical therapy is best, but also when it should be performed, and which therapy would benefit the very old best.
An inclusion of patients in a surgical RCT on ICH depends on several variables, not only age. Some prognostic factors, like the volume of the intracerebral hematomas, are not available in our data. However, this variable is not essential for an analysis of upper age limits. The rate of patients with disabilities is significantly higher in the very old compared to younger patients; this is an exclusion criterion in several surgical trials on ICH. In this case we have overestimated the amount of excluded patients in our analysis.
Conclusions
Patients with spontaneous ICH outside surgical RCT on ICH in central Europe are older compared to the published study populations. Currently, no specific surgical trial for the very old with spontaneous ICH exists, and it is questionable whether that would occur. This should be noted if results from surgical RCTs for spontaneous ICH are transferred to clinical practice among the very old.
Acknowledgements
Thanks to the Hessian Stroke Working Group, who administered data on stroke patients in the context of external quality assurance; participants are listed at www.gqhnet.de.
References
- 1.Feigin V L, Lawes C M, Bennett D A, Barker-Collo S L, Parag V. (2009) Worldwide stroke incidence and early case fatality reported in 56 population-based studies: A systematic review. , The Lancet. Neurology 8, 355-369.
- 2.van Asch CJ, Luitse M J, Rinkel G J, Tweel I van der, Algra A. (2010) Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: A systematic review and meta-analysis. Lancet neurology. 9, 167-176.
- 3.Stein M, Misselwitz B, Hamann G F, Scharbrodt W, Schummer D I. (2012) Intracerebral hemorrhage in the very old: Future demographic trends of an aging population. , Stroke 43, 1126-1128.
- 4.McMurdo M E, Witham M D, Gillespie N D. (2005) Including older people in clinical research. , Bmj 331, 1036-1037.
- 5.Arboix A, Garcia-Eroles L, Massons J, Oliveres M, Targa C. (2000) Acute stroke in very old people: Clinical features and predictors of in-hospital mortality. , Journal of the American Geriatrics Society 48, 36-41.
- 6.McKissock W, Richardson W, Taylor J. (1961) Primary intracerebral haemorrhage: A controlled trial of surgical and conservative treatment in 180 unselected cases. The Lancet. 278, 221-226.
- 7.Auer L M, Deinsberger W, Niederkorn K, Gell G, Kleinert R. (1989) Endoscopic surgery versus medical treatment for spontaneous intracerebral hematoma: A randomized study. , Journal of neurosurgery 70, 530-535.
- 8.Wang W Z, Jiang B, Liu H M, Li D, Lu C Z. (2009) Minimally invasive craniopuncture therapy vs. Conservative treatment for spontaneous intracerebral hemorrhage: Results from a randomized clinical trial in china. International journal of stroke : official journal of the International Stroke Society 4, 11-16.
- 9.Sun H, Liu H, Li D, Liu L, Yang J. (2010) An effective treatment for cerebral hemorrhage: Minimally invasive craniopuncture combined with urokinase infusion therapy. , Neurol Res 32, 371-377.
- 10.Stolz E, Hamann G F, Kaps M, Misselwitz B. (2011) Regional differences in acute stroke admission and thrombolysis rates in the german federal state of hesse. , Deutsches Arzteblatt international 108, 607-611.
- 11.Juvela S, Heiskanen O, Poranen A, Valtonen S, Kuurne T. (1989) The treatment of spontaneous intracerebral hemorrhage. A prospective randomized trial of surgical and conservative treatment. , J Neurosurg 70, 755-758.
- 12.Teernstra O P, Evers S M, Lodder J, Leffers P, Franke C L. (2003) Stereotactic treatment of intracerebral hematoma by means of a plasminogen activator: A multicenter randomized controlled trial (sichpa). , Stroke 34, 968-974.
- 13.Mendelow A D, Gregson B A, Rowan E N, Murray G D, Gholkar A. (2013) Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (stich ii): A randomised trial. , Lancet 382, 397-408.
- 14.Morgenstern L B, Frankowski R F, Shedden P, Pasteur W, Grotta J C. (1998) Surgical treatment for intracerebral hemorrhage (stich): A single-center, randomized clinical trial. , Neurology 51, 1359-1363.
- 15.Zuccarello M, Brott T, Derex L, Kothari R, Sauerbeck L. (1999) Early surgical treatment for supratentorial intracerebral hemorrhage: A randomized feasibility study. , Stroke 30, 1833-1839.
- 16.Mendelow A D, Gregson B A, Fernandes H M, Murray G D, Teasdale G M. (2005) Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the international surgical trial in intracerebral haemorrhage (stich): A randomised trial. , Lancet 365, 387-397.
- 17.Batjer H H, Reisch J S, Allen B C, Plaizier L J, Su C J. (1990) Failure of surgery to improve outcome in hypertensive putaminal hemorrhage. A prospective randomized trial. , Arch Neurol 47, 1103-1106.
- 18.Tan S H, Ng P Y, Yeo T T, Wong S H, Ong P L. (2001) Hypertensive basal ganglia hemorrhage: A prospective study comparing surgical and nonsurgical management. Surgical neurology. 56, 287-292.
- 19.Pantazis G, Tsitsopoulos P, Mihas C, Katsiva V, Stavrianos V. (2006) Early surgical treatment vs conservative management for spontaneous supratentorial intracerebral hematomas: A prospective randomized study. Surgical neurology. 66, 492-501.
- 20.Hattori N, Katayama Y, Maya Y, Gatherer A. (2006) Impact of stereotactic hematoma evacuation on medical costs during the chronic period in patients with spontaneous putaminal hemorrhage: A randomized study. , Surgical 65, 429-435.
- 21.Cho D Y, Chen C C, Chang C S, Lee W Y, Tso M. (2006) Endoscopic surgery for spontaneous basal ganglia hemorrhage: Comparing endoscopic surgery, stereotactic aspiration, and craniotomy in noncomatose patients. Surgical neurology. 65, 547-555.
Cited by (1)
- 1.Stein Marco, 2021, , , (), 143, 10.1007/978-3-662-60354-3_11
