Abstract
Background:
Optic nerve head drusen are acellular hyaline deposits located anterior to the lamina cribrosa, frequently associated with visual field defects. Sometimes rapid worsening of vision may occur due to complications such as acute vascular events, choroidal neovascularization, or serous maculopathy.
Case Presentation:
Although there are no proven treatments for Optic nerve head drusen associated field loss, we present the case of a patient with Optic nerve head drusen and bilateral rapid progression of visual field loss that has stabilized on intraocular pressure lowering medication. This suggests a role for IOP-mediated retinal ganglion cell loss in this individual. The mechanism of progressive Optic nerve head drusen associated field loss is poorly understood, however experimental glaucoma models and human in vivo imaging studies have shown that structural differences within the optic nerve head are likely to contribute to individual susceptibility to IOP-mediated damage.
Conclusion:
We propose that eyes with Optic nerve head drusen may be less able to dampen IOP mediated stress, contributing to loss of retinal ganglion cells in some patients.
Author Contributions
Academic Editor: Singh K, Guru Nanak Eye Centre, Maulana Azad Medical College and Affiliated Hospitals
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2016 Karim El-Assal, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Optic nerve head drusen (ONHD) are acellular hyaline deposits of calcium, amino and nucleic acids, and mucopolysaccharides, found in the prelaminar portion of the optic nerve head believed to arise from altered retinal ganglion cell (RGC) axoplasmic flow1,2. First described by Liebreich in 18683, ONHD have been reported to affect 0.4% to 20.4% of individuals.4ONHD may be difficult to detect in young patients, or confused with papilledema, as they tend to be buried beneath the surface of the optic disc, close to the lamina cribrosa5,6. Over time ONHD tend to enlarge and become located closer to the surface of the ONH, which acquires an irregular lumpy appearance7. While these changes typically occur slowly, in some patients ONHD can enlarge rapidly8.
Although ONHD are normally asymptomatic and detected incidentally, they are associated with visual field defects in up to 24% to 87% of affected adults1,7,9. The mechanism of ONHD-related visual field loss is poorly understood, however drusen typically enlarge slowly throughout life and this is often associated with slow progression of visual field loss1,7,9. Superficial ONHD are also more commonly associated with visual field defects than those located more deeply2. Despite the lack of quantitative longitudinal imaging studies, it is therefore seems likely that progressive field loss in patients with ONHD is due to progressive drusen enlargement.
Occasionally ONHD may be associated with rapid worsening of vision, with reported complications including choroidal neovascularization, serous maculopathy or acute vascular events such as central retinal arterial or venous occlusion or non-arteritic ischemic optic neuropathy10, 11, 12. In the absence of these complications, there are no proven treatments for visual loss associated with ONHD.
It is hypothesized that ONHD-related visual field loss may occur as a result of mechanical stress on RGC axons within the prelaminar scleral canal resulting in retrograde axonal degradation and RGC death1,13. The mechanism of ONHD-related visual loss may therefore share some characteristics with glaucoma, in which RGC death is also believed to be due to interruption of retrograde axoplasmic flow14. This raises the possibility that treatments to lower intraocular pressure (IOP) might have a role in slowing visual field loss in patients with progressive ONHD-related field loss.
We present the case of a patient with bilateral ONHD with rapidly progressing visual field loss, who has had slowing of disease progression with topical IOP-lowering medication. The patient had extensive investigations to rule of out possible co-existing conditions that might have contributed to visual loss.
Case Presentation
Case History
A 60-year-old female was referred in September 2012 after she complained of slowly worsening vision in the right over the previous year. She had attended her community optometrist who had noted bilateral optic disc swelling. The patient was a low hypermetrope but had experienced no other previous ophthalmic problems. She did however have a history of treated type 2 diabetes mellitus, hypertension and mild asthma. She had not required any systemic steroids for her asthma and there was no significant family history of eye disease.
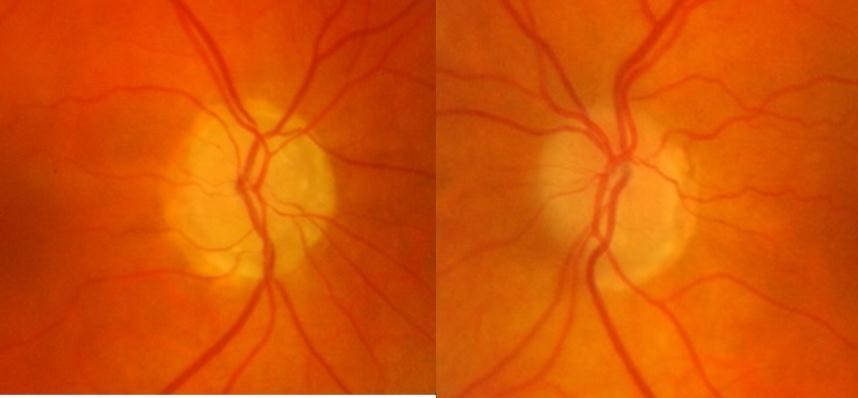
At presentation, her best-corrected visual acuity was 6/6 in either eye, colour vision was normal and she had normal pupil reactions. IOP was 14mmHg in each eye on applanation tonometry and her central corneal thickness measured 491um and 498um in right and left eyes respectively. Anterior segment examination was unremarkable and she had open drainage angles on gonioscopy. Dilated fundus examination showed bilateral ONHD (Figure 1),
which were confirmed by fundus autofluorescence and B scan ultrasonography(Figure 2).(Figure 3) The optic discs were not abnormally excavated and no localised retinal nerve fibre layer (RNFL) defects were visible, however there was some inferior beta zone peripapillary atrophy in both eyes. Standard automated perimetry (SAP), which was of good reliability, showed significant decreased sensitivity in both eyes but more advanced in the right eye (Figure 4) with a mean deviation (MD) of -15.57 dB in the right eye and -3.13 dB in the left. Due to the disc swelling and marked visual field defects she underwent MRI imaging of her brain and visual pathways, which was normal.
Figure 2.Fundus autofluorescence
The visual field defects were thought to be due to the ONHD and as there is no proven treatment for this condition, the patient was counselled regarding the possibility that the field defects may progress. Follow up was arranged for confirmatory visual field testing in 3 months. At the return visit her visual field was slightly worse, with MD of -18.41 dB and -9.59 dB in the right and left eye respectively (Figure 4). The patient was observed to have good fixation and concentration throughout visual field testing. Her IOP remained 14 mmHg in both eyes. Due to her thin corneas, and the possibility that ONHD might be masking glaucomatous changes to the ONH, at this visit she was commenced on a prostaglandin analogue eye drop to both eyes. Treatment resulted in a reduction in IOP to 10 mmHg in the right eye (29% reduction from baseline) and 11mmHg in the left (21% reduction from baseline).
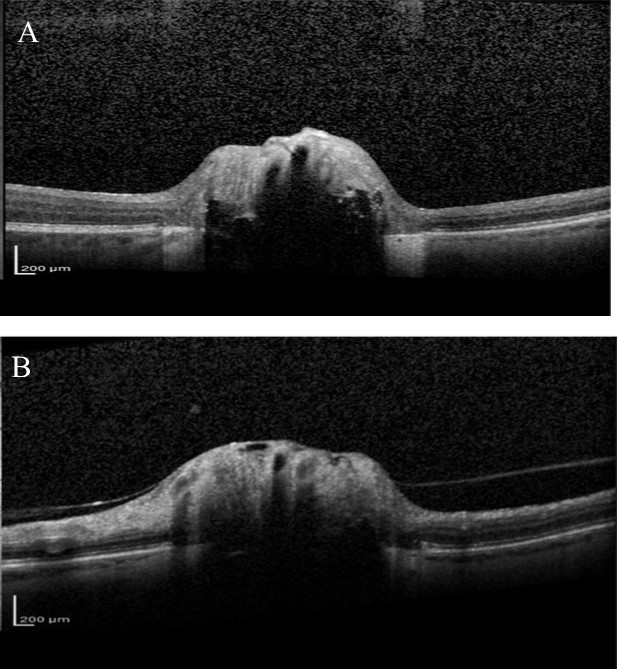
Over the next 6 months she felt the deterioration in vision in right eye had stabilised but complained that the vision in her left eye was gradually worsening. Visual acuity remained good but visual field testing confirmed a deterioration showing there had been a marked decline in the left eye visual field with MD deterioration from -9.59 to -24.01(Figure 4). Blood tests were taken for common Leber’s hereditary optic neuropathy mutations, which were negative. Vitamin B12 and folate levels were within normal range. Fundus fluorescein angiography (FFA) was also performed, looking for possible associated anterior ischaemic optic neuropathy (AION), but no evidence of late leakage was found. OCT examination of the RNFL was also performed and showed significant thinning in both eyes (Figure 5). Average RNFL thickness was only 57 um in the right eye but measured 87 um in the left, although the inferotemporal sector was outside normal limits in the left eye. Enhanced depth imaging (EDI) OCT was also performed to better ascertain the location and extent of the ONHD (Figure 6). Electro-diagnostic tests showed reduced amplitudes and prolonged latency on the visual evoked potential (VEP) for both eyes, with a normal electroretinogram. The reduced amplitude and prolonged latency on the VEP was suggestive of a non-demyelinating optic neuropathy.
Given the further progression in visual field, the decision was taken to escalate IOP-lowering treatment and the patient was commenced on a topical carbonic anhydrase inhibitor, beta-blocker and an alpha-2-agonist, in addition to the prostaglandin analogue. This resulted in a further decrease in IOP, to 9 mmHg in each eye (36% reduction from baseline). The patient tolerated the treatment well and decided to continue with this treatment and after 1 year, feels her vision has remained stable, which was confirmed with repeat perimetry (MD right eye -23.75 and -23.05 left eye).
Discussion
Despite rapid visual field loss over the preceding 12 months, the patient described in this case showed subjective and objective stabilization following instigation of IOP lowering medication. It is important to acknowledge that this is a single case and other patients may not respond similarly, however the cases raises the possibility that IOP-lowering may have a role in some patients with ONHD.
The mechanism of visual field loss in ONHD is poorly understood, however, it has been postulated that enlarging drusen may damage RGC axons directly through mechanical insult, or indirectly by compressing ONH vasculature leading to ischemia12. Therefore the suggested mechanism of visual loss in ONHD is not dissimilar from that believed to occur in the pathogenesis of glaucoma14. Progressive visual field loss is not infrequent15 with Mustonen and colleagues reporting visual field progression in 22% of patients with ONHD16. The rate of ONHD-related visual field loss is usually slow17, however our patient experienced a fast rate with a change in MD from -3.13 to -17.3 in the left eye and from -15.57 to – 22.50 in the right eye in just over 12 months.
Most previous cases of rapid visual loss in ONHD have been associated with an acute vascular event or choroidal neovascualarization. We performed FFA to investigate for secondary causes of visual loss and to look for disc leakage, however FFA was normal. Reduced amplitude and prolonged latency on the VEP, with a normal electroretinogram, suggested visual loss was due to an optic neuropathy. This is in agreement with previous studies, which have shown patients with ONHD to have prolonged latency on VEPs18, 19, 20, a finding consistent with a mechanical compressive mechanism of axonal damage. The prolonged latency and reduced amplitude in our patient was likely due to the marked extent of the nerve damage 21. Although our patient had marked ONHD, as evident from the EDI-OCT, B scan ultrasonography and autofluorescence, it is not possible to be certain that ONHD was the sole cause of visual deterioration. It is conceivable that she also had coexisting normal tension glaucoma, however the rapid deterioration in visual field would not be characteristic of this condition. Given the difficulty of diagnosing glaucoma in patients with ONHD, and the possible increased risk of IOP-mediated RGC injury in an already compromised eye, we opted to treat our patient with topical IOP lowering medication7,22.
Discerning whether visual field loss is due to glaucoma or ONHD is challenging in eyes with confirmed ONHD, as drusen distort normal ONH anatomy, making detection of glaucomatous structural changes problematic. Furthermore, the pattern of visual field loss is not likely to be helpful in differentiating glaucomatous and ONHD-related visual losses, as the anatomical location of the pathology is similar. Although ONHD can lead to blind spot enlargement, which is not characteristic of glaucoma, drusen can also commonly result in glaucomatous-type defects such as nerve fibre bundle defects16,23.
Improvements in technologies such as EDI-OCT and Swept Source OCT might enable better identification of glaucomatous changes in eyes with ONHD as they allow imaging of deep ocular structures13,24,25. The ability of EDI-OCT to image structures 500-800 um deeper than conventional OCT allows the posterior limit of the drusen to be imaged13. Several studies have recently shown that using EDI-OCT or Swept Source OCT it is possible to visualise the extent of ONHD and to examine the thickness of neighbouring neural tissue13. Our patient had marked RNFL loss on SDOCT, particularly in the right eye. The RNFL thickness was outside normal limits, however, normative databases should be interpreted with caution in eyes with ONHD particularly as the risk of segmentation errors is likely to be higher due to abnormal anatomy. Although glaucomatous RNFL thinning characteristically involves the inferior-temporal and superior-temporal circumpapillary RNFL, the pattern of RNFL loss may not help differentiate whether this is related to ONHD or glaucoma as the location of RNFL defects associated with ONHD is likely to vary depending on location of the ONHD.
There is little previous literature regarding the coexistence of glaucoma and ONHD, however, Samples and colleagues reported a small cases series of 5 patients22. The authors concluded that keeping the IOP as low as possible was advisable due to the difficulty of monitoring optic nerve changes. Mamikonian and colleagues examined 35 eyes of 21 patients with ONHD, of which 8 eyes also had glaucoma. Patients with glaucoma were found to have significantly reduced ocular blood flow compared to controls, suggesting ocular blood flow might be useful in differentiating these conditions26. However, some eyes with ONHD also have impaired blood flow as evident from the increased risk of ocular vascular events. In an additional manuscript, Roh and colleagues presented 2 cases of combined ONHD and glaucoma where both patients had significant RNFL thinning on time domain-OCT. One of the patients had glaucoma in both eyes but ONHD only in one eye. RNFL thinning was more pronounced in the eye with ONHD, which was suggested to be a result of a possible synergistic effect of both conditions in damaging the RNFL27.
An interesting consideration is whether ONHD might increase the likelihood of glaucoma. It has been proposed that structural differences within the ONH could contribute to individual susceptibility to IOP-mediated damage in glaucoma. Eyes with a particular combination of ONH anatomy, mechanical strength or blood supply may be more susceptible to damage at normal levels of IOP, whereas others may be less vulnerable28. Recent studies have shown that IOP elevation results in displacement of the lamina cribrosa and expansion of the scleral canal 29. Eyes that are less able to dampen IOP changes are likely to be higher risk of RGC loss due to mechanical pressure on RGC axons passing through the lamina pores. It is likely that eyes with ONHD may be less able to dampen IOP changes, potentially exposing RGCs to greater mechanical strain, perhaps at levels of IOP that are normally physiological. Other authors have recognised the importance of biomechanical factors in ONHD-related visual loss and it has been proposed that patients with ONHD and congenitally small scleral canals might be at higher risk of visual loss due to lack of space for enlarging drusen30.
Conclusion
In conclusion, rapid visual loss is uncommon in patients with ONHD but may due to an acute vascular event, choroidal neovascular membrane or non-arteritic ischemic optic neuropathy. Our patient had none of the above, but nonetheless had rapid loss of visual field over 12 months following initial presentation. Although we could not conclusively establish a diagnosis of glaucoma, objective and subjective measures of vision have stabilised since she was commenced on IOP-lowering topical medication. Structural differences within the ONH are likely to contribute to individual susceptibility to IOP-mediated damage and we propose that ONH with ONHD may be less able to dampen IOP stress and strain contributing to loss of RGCs in these patients. IOP-lowering should be considered as a treatment option if other causes of visual loss have been excluded.
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
List of Abreviations
AION: Anterior ischaemic optic neuropathy
EDI: Enhanced depth imaging
FFA: Fundus fluorescein angiography
IOP: Intraocular pressure
MD: Mean deviation
ONHD: Optic nerve head drusen
RGC: Retinal ganglion cell
RNFL: Retinal nerve fibre layer
SAP: Standard automated perimetry
VEP: Visual evoked potential
Authors’ Contributions
KE: Data collection, reviewing the literature, preparing the illustrations and figures and drafting the manuscript
AT: Revising the manuscript critically for important intellectual content and giving final approval of the version to be published
Supported in part by:
NHS Scotland Career Research Fellowship (AJT)
The author(s) have made the following disclosure(s): A.J.T. – research support from Heidelberg Engineering
References
- 1.B J Katz, H D Pomeranz. (2006) Visual field defects and retinal nerve fiber layer defects in eyes with buried optic nerve drusen,” (eng), American journal of ophthalmology. 141(2), 248-253.
- 2.K M Lee, S J Woo, Hwang J-M. (2011) Differentiation of optic nerve head drusen and optic disc edema with spectral-domain optical coherence tomography,” (eng), Ophthalmology. 118(5), 971-977.
- 3.Liebrich R.Contribution to discussion on Iwanoff A. Ueber Neuritis optica. Klin Monatsbl Augenheilkd1868,6: 426–7”.
- 4.D Z Law, Yang Francine Pei Lin, Teoh Stephen.Charn Beng “Case report of optic disc drusen with simultaneous peripapillary subretinal hemorrhage and central retinal vein occlusion,” (eng), Case reports in ophthalmological medicine,2014,2014,p.156178.
- 5.E M Arbabi, T E Fearnley, Z I Carrim. (2010) Drusen and the misleading optic disc,” (eng), Practical neurology. 10(1), 27-30.
- 6.Erkkilä H, Laatikainen L. (1979) Characteristics of optic disc in healthy school children,” (eng), Acta ophthalmologica. 57(5), 914-921.
- 7.Auw-Haedrich C, Staubach F, Witschel H. (2002) Optic disk drusen,” (eng), Survey of ophthalmology. 47(6), 515-532.
- 8.R Du Seo, S H Park. (2015) Case of rapidly progressing buried optic nerve head drusen,” (eng), JAMA ophthalmology. 133(2), 143467.
- 9.Sato T, Mrejen S, R F Spaide. (2013) Multimodal imaging of optic disc drusen,” (eng), American journal of ophthalmology. 156(2), 275-282.
- 10.T M Grippo, W A Shihadeh, Schargus M, Gramer E, Tello C et al. (2008) Optic nerve head drusen and visual field loss in normotensive and hypertensive eyes,” (eng). , Journal of glaucoma 17(2), 100-104.
- 11.S G Farah, A M Mansour.Central retinal artery occlusion and optic disc drusen,” (eng). , Eye (London, England),1998,12(Pt3a),pp.480–482
- 12.Kamath G G, Prasad S, R P Phillips. (2000) Bilateral anterior ischaemic optic neuropathy due to optic disc drusen,” (eng), European journal of ophthalmology. 10(4), 341-343.
- 13.A L Silverman, A J Tatham, F A Medeiros, R N Weinreb. (2014) Assessment of optic nerve head drusen using enhanced depth imaging and swept source optical coherence tomography,” (eng). , Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology 34(2), 198-205.
- 14.R N Weinreb, Aung T, F A Medeiros. (2014) The pathophysiology and treatment of glaucoma: a review,” (eng), JAMA. 311(1), 1901-1911.
- 15.P J Savino. (1979) A Clinical Analysis of Pseudopapilledema,” Archives of ophthalmology. 97(1), 71.
- 16.Mustonen E. (1983) Pseudopapilloedema with and without verified optic disc drusen. A clinical analysis II: visual fields,” (eng), Acta ophthalmologica. 61(6), 1057-1066.
- 17.A G Lee, M B Zimmerman. (2005) The rate of visual field loss in optic nerve head drusen,” (eng), American journal of ophthalmology. 139(6), 1062-1066.
- 18.R A Stevens, Newman N M. (1981) Abnormal visual-evoked potentials from eyes with optic nerve head drusen,” (eng), American journal of ophthalmology. 92(6), 857-862.
- 19.G B Scholl, H S Song, D E Winkler, S H Wray. (1992) The pattern visual evoked potential and pattern electroretinogram in drusen-associated optic neuropathy,” (eng), Archives of ophthalmology. 110(1), 75-81.
- 20.T M Grippo, Ezon I, F N Kanadani, Wangsupadilok B, Tello C et al. (2009) The effects of optic disc drusen on the latency of the pattern-reversal checkerboard and multifocal visual evoked potentials,” (eng), Investigative ophthalmology & visual science. 50(9), 4199-4204.
- 21.Kothari R, Bokariya P, Singh S, Singh R. (2013) Significance of Visual Evoked Potentials in the Assessment of Visual Field Defects in Primary Open-Angle Glaucoma:. , A Review,” Neuroscience Journal 2013(1), 1-6.
- 22.J R Samples, M van Buskirk, W T Shults, Dyk H J Van. (1985) Optic nerve head drusen and glaucoma,” (eng), Archives of ophthalmology. 103(11), 1678-1680.
- 23.J M Wilkins, H D Pomeranz. (2004) Visual manifestations of visible and buried optic disc drusen,” (eng). , Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society 24(2), 125-129.
- 24.R F Spaide, Koizumi H, M C Pozzoni, M C Pozonni. (2008) Enhanced depth imaging spectral-domain optical coherence tomography,” (eng), American journal of ophthalmology. 146(4), 496-500.
- 25.K Y Merchant, Su D, S C Park, Qayum S, Banik R et al. (2013) Enhanced depth imaging optical coherence tomography of optic nerve head drusen,” (eng), Ophthalmology. 120(7), 1409-1414.
- 26.V R Mamikonian, N S Galoian, N L Sheremet, Kazarian E E, S I Kharlap. (2013) [Differentiation of concomitant glaucomatous optic neuropathy in optic disc drusen],” (rus), Vestnik oftalmologii. , OA Shemeleva-Demir, A A 129(5), 68-72.
- 27.Roh S, R J Noecker, J S Schuman. (1997) Evaluation of coexisting optic nerve head drusen and glaucoma with optical coherence tomography,” (eng), Ophthalmology. 104(7), 1138-1144.
- 28.C F Burgoyne, J C Downs, A J Bellezza, J-K F Suh, R T Hart. (2005) The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage,” (eng), Progress in retinal and eye research. 24(1), 39-73.
Cited by (2)
- 1.Radenković Marija, Stanković-Babić Gordana, Đorđević-Jocić Jasmina, Trenkić Marija, Cekić Sonja, et al, 2022, The influence of topical antiglaucoma drugs on the reduction of the decrease of visual field sensitivity due to optic nerve head drusen: Case report, Medicinski casopis, 56(1), 38, 10.5937/mckg56-34895
- 2.Radenković Marija, 2019, , , (), 10.5772/intechopen.80278