Abstract
Background:
To measure the affected subepidermal nerve sensation with a combination of current perception threshold (CPT) and Semmes-Weinstein monofilament (SWM) testing under pain areas with herpetic ophthalmic neuralgia (HON).
Methods:
One hundred fifty-five subjects with HON and 30 age-matched volunteers were recruited for the study.
Results:
The combined SWM and CPT testing yielded significantly abnormal responses at the painful sites for the patients with HON compared to those responses of the normal controls (p< 0.05). The SWM and CPT tests were able to distinguish local hypoesthesia of pain areas when the cut-off values were 0.135g, 53 (5Hz), 66 (250Hz) and 199 (2,000Hz). The ratio of the painful site to the mirror site indicated there were significant differences in the CPT ratio at 250Hz and 2,000Hz among different pain characteristic groups. Post hoc tests showed significant differences in CPT250Hz between itching and lancinating pain (p=0.006), itching and allodynia (p=0.002), and burning pain and allodynia (p=0.042). There were significant differences in CPT2000Hz between deep pain and allodynia (p=0.001) and itching and allodynia (p=0.014).
Conclusion:
CPT and SWM testing can probably be used as semi-quantitative indicators to represent the neural function at the local skin of pain area with HON.
Author Contributions
Academic Editor: Jyotsna Maram, Doheny Eye Institute, USA, Email: [email protected]
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 Gang
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Herpes zoster (HZ) is a common, painful and debilitating condition caused by a reactivation of the varicella-zoster virus (VZV) from a latent infection of sensory ganglia1, 2. Among the cranial nerves affected by VZV, herpes zoster ophthalmicus (HZO) which originated from the ophthalmic division (V1) of the trigeminal nerve (V) 3, 4, 5, is the most common and accounts for 10–25% of all HZ infection 6, 7, followed by thoracic dermatomes. Herpetic ophthalmic neuralgia (HON), a persistent painful condition that can become chronic, is one of the most common complications of HZO 5, 8. HON is likely to be due to nociceptive response to local inflammation and tissue damage stimulation, which are manifested neurologically as pain9. Symptoms are non-specific and range from itching to an intense burning sensation10. Consequently, V1 is one of the most common sites of postherpetic neuralgia (PHN) in the elderly3, 11, and this condition can severely impact the quality of life (QoL) in these patients 12.
Although the development of PHN would most likely be neural injury13, no previous study has provided clinical evidence of the severity of neural damage. The persistence of local subcutaneous pain after the onset of vesicles implied that the peripheral mechanisms of cutaneous innervations, such as the subepidermal nerve plexus, epidermal nerve fibers and terminals, played an important role in the pathogenesis of HON7, 14, but little is known about how the phenotypic and functional changes of the affected pain area occur.
A sensitive, reliable, and quantitative test of V1 function in HON may be extremely essential to broaden our knowledge of the underlying pain-generating mechanisms, diagnosing HON and monitoring of therapeutic outcomes. Nerve conduction techniques allow direct measurement of the sensory nerve action potential and sensory conduction velocity, thus providing a quantitative measure of the functioning large fibers in the nerve. V1 is a purely sensory nerve that has a large concentration of unmyelinated fibers and consequently, a lower A/C fiber ratio when compared with V2 and V3 15. This ratio of fibers makes it difficult to evaluate the ophthalmic neurosensory functions by sensory conduction velocity. Von Frey's hairs only have moderate sensitivity and specificity for diagnosis of neurophysiological and clinical neuropathy16. Quantitative sensory testing (QST) relies on analysis of an individual's response to external stimuli, reflecting the integrity of the peripheral nervous system and the sensory pathway17. The sensory pathway cannot be assessed in isolation from the affective and cognitive characteristics of patients or testers. Many variables potentially affect the reliability and reproducibility of QST, which after all, is designed for the testing of individuals by other individuals. Relative comparisons across body regions do not offer advantages over absolute reference values. There is little report to detect V1 area neurological function. Measuring the current perception threshold (CPT) of sensory nerves performed by the neurometer device could obtain quantified sensory nerve function18, 19. This test reportedly achieves differential neuro-excitatory effects by using different frequencies of an electrical sine wave stimulus. CPT testing has been validated and well-documented in peripheral neuropathy resulting from several different etiologies20, 21, 22. However, studies utilizing CPT testing for evaluation of cutaneous innervations in HON patients are limited 23. The aims of the present study were to measure the affected subepidermal nerve sensation with a combination of CPT and Semmes-Weinstein monofilament (SWM) testing to detect sensory function changes in the cutaneous innervations under pain areas with HON.
Methods
The study was conducted in accordance with the Helsinki Declaration and was approved by the Research Ethics Board of Shanghai Tenth People's Hospital (2014─49). This research was performed from March 2014 to December 2016 in the Department of Rehabilitation Medicine. Using a statistical sampling formula with α = 0.05, β =0.2, p1 = 0.86 and p2= 0.96, the number of samples was 150 people who were suffering from HON.
Participants
All subjects were at least 50 years of age, and the sample consisted of healthy volunteers and patients with HON who were recruited from the Shanghai Tenth People's Hospital. The reference standard for the diagnosis of HON was Dworkin`s diagnostic criteria12: The subjects were suffering cutaneous and/or subcutaneous pain in the unilateral V1 region temporally and spatially associated with their rash; had a worst pain score of four or higher on an 11-point pain intensity numerical rating scale (NRS) in the past 24 hours; and had pain that persisted more than 15 days after rash onset. Topical agents or/and oral medications, including antidepressants, antiepileptics, and antiarrhythmics were discontinued 10 days before the initiation of the study. Exclusion criteria were the subjects (including the volunteers) having a history of neurological or dermatological disease other than HON, cognitive impairment, or systemic disease, such as diabetes mellitus or renal failure. The subjects with prodromal pain preceding the appearance of a rash were also excluded.
Study Protocol Overview
After obtaining Institutional Review Board Approval, one hundred fifty-five subjects with pain and 30 aged-matched healthy volunteers were enrolled in the study. All subjects who fulfilled the inclusion criteria, provided written informed consent. The subjects with HON were divided into five groups by pain duration. The HON-30 group had pain duration more than 15 days and less than 30 days, the HON-60 group had pain more than or equal to 30 days and less than 60 days, the HON-90 group had pain no more than 90 days, the HON-120 group had pain no more than 120 days, and the HON>120 group had pain more than 120 days after rash onset. All subjects underwent a structured interview to assess the clinical and phenomenological characteristics of their pain: descriptive characteristics and pain intensity on the NRS.
The study was conducted in a quiet room to encourage the subject to focus on the test. The temperature of the testing room was maintained at 20℃-22℃, and the humidity was approximately 60%. Light and heat were blocked and indoor airflow was maintained constantly. The procedure was explained to the patient before the test. All testing was performed with subjects in stationary positions (supine) to reduce the possibility of position interference while being tested. One day before examination, patients were asked to refrain from physical therapy, thermotherapy, and electro-physiologic study.
Clinical examination
All subjects underwent a general and neurological examination. Sensory disturbances and pain were carefully assessed. Before testing, all patients were interviewed and asked to indicate the severity of their current pain, to rate the pain intensity on an 11-point NRS ranging from 0 (no pain) to 10 (worst possible pain) and to describe their predominant pain in one of the following categories: constant pain, deep pain, burning pain, paroxysmal pain, lancinating pain, allodynia, paresthesia, dysesthesia, hyperalgesia, and itching12, 24.
SWM Testing
The SWM test using Touch Test Sensory Evaluators (Anaesthesio® Dr. med. Martin Bloch, Düsseldorf, Germany) examines the cutaneous pressure threshold by a reproducible buckling stress filament that exerts a constant force onto the supraorbital area for 3–5 seconds. Monofilament testing was performed by the third author, who had received the necessary training on the use of monofilaments. The monofilament was pressed on the supraorbital area as far as the monofilament could be bent until the subject responded to the stimulus, while the subject’s eyes were closed. At each point, the test was repeated three times. The threshold was defined as the lightest filament that the subject responded to correctly in at least two of the three trials. The filaments are labeled with a numerical marking, which is a log base ten of the force in tenths of milligrams25.
Neurophysiological Testing Procedures
The neurometer (Neurotron, Baltimore, USA) electrodiagnostic testing device uses a standardized, automated procedure26, 27. CPT testing was performed by the second author, who had received the necessary training on the use of neurometer. After skin preparation with an alcohol swab, the paired electrode was attached to the test site with a 5-mm distance between the outer rims of each electrode. Stimulations of a constant sinusoidal electrical current with 3 different frequencies (5 Hz, 250 Hz and 2,000Hz) were delivered to the test site. At each frequency, the current was slowly increased from 0.01 mA until the subject reported a sensation and pressed a button. The test sites of volunteers were at the bilateral supraorbital area. As a mirror site (MS), the first site was placed contralateral to and at the level of the supraorbital area to determine a reference value for each individual. The painful site (PS) at HZ-affected supraorbital area was marked for quantitative tests of sensory function, avoiding open lesions. To initially familiarize the procedure, the test was performed on the MS to the PS in the subjects with HON. At each point, the test was repeated two times.
Statistical Analysis
An unpaired (2-tailed) t test was used to evaluate differences of the mean for SWM and CPT values between the female group and male group. Paired t tests were used to test the difference between the mean for SWM and CPT values obtained for different sides (left-right). Receiver operating characteristic (ROC) analysis was used to evaluate the predictive value of each biomarker separately for the disease progression of HON. The ROC curve was used to illustrate the sensitivity versus 1-specificity of SWM and CPT values along a sequence of cut-points with both healthy subjects and subjects with HON. The highest area under the curve (AUC) and Youden index were used to select the cut-off value of the biomarker’s measurement. The median and inter-quartile ranges (IQR) were calculated for each item, the Kruskal-Wallis H test was performed to compare the PS/MS ratio among groups and the Dunn-Bonferroni`s tests were used for post-hoc comparisons. P-values less than 0.05 were considered statistically significant. All tests were performed with R software.
Results
Variability of the SWM and CPTs
The demographic characteristics of the study population are shown in Table 1. For each volunteer, the SWM and CPTs were tested from both the left and right sides. The means and 95% confidence intervals (CI) of the SWM and CPTs are shown in Table 2. The first step was to investigate whether there were differences in the SWM and CPTs related to gender and the different sides in which the distributions exhibited Gaussian distributions. The descriptive values and results are shown in Table 2. There were no significant differences when comparing the SWM and CPTs in men and women. The differences for the different (left-right) sides were not statistically significant in the SWM and CPT readings at each frequency according to paired t tests.
Table 1. Demographic characteristics of the 30 healthy control subjects and 155 HON patients| Female | Male | |||||||
| n | Age (y) | Duration(days) | Pain intensity (NRS) | n | Age (y) | Duration(days) | Pain intensity (NRS) | |
| Healthy controls | 15 | 63.7(6.9) | 15 | 64.7(7.0) | ||||
| HON-30days | 13 | 64.9(7.0) | 21.6(6.6) | 8.1(1.3) | 16 | 66.2(8.1) | 23.0(5.3) | 7.8(1.9) |
| HON-60days | 13 | 65.9(4.9) | 46.8(10.7) | 7.2(2.1) | 18 | 66.6(5.2) | 51.7(8.1) | 7.2(1.7) |
| HON-90days | 17 | 63.9(6.1) | 75.7(18.6) | 7.5(2.0) | 15 | 67.1(5.7) | 77.5(17.1) | 6.9(2.2) |
| HON-120days | 15 | 64.6(5.8) | 115.0(8.7) | 6.7(1.8) | 13 | 66.8(7.1) | 113.9(9.8) | 7.3(2.3) |
| HON>120days | 18 | 60.1(4.6) | 226.0(79.3) | 6.8(1.6) | 17 | 59.2(5.4) | 237.1(81.5) | 7.0(2.0) |
| Measurement | Mean(SD) | 95% CI | Left / Right | Male / Female | |||
| Mean(SD) | p | Mean(SD) | p | ||||
| SWM (g) | 0.118(0.077) | 0.099 to 0.138 | 0.123(0.077) / 0.112(0.077) | 0.55 | 0.127(0.086) / 0.109(0.067) | 0.37 | |
| CPT 5Hz(mA×100) | 34.38(17.17) | 30.40 to 38.62 | 34.3(15.9) / 34.4(18.6) | 0.98 | 30.1(16.1) / 38.6(17.4) | 0.05 | |
| CPT 250Hz(mA×100) | 44.33(15.28) | 40.73 to 48.00 | 45.7(15.3) / 43.0(15.4) | 0.49 | 43.6(18.6) / 45.1(11.2) | 0.70 | |
| CPT 2000Hz(mA×100) | 133.53(32.73) | 125.10 to 142.15 | 131.2(32.4) / 135.9(33.5) | 0.58 | 130.5(34.2) / 136.5(31.5) | 0.48 | |
Although the SWM and CPTs values had high standard deviation in the healthy controls, no significant differences were found between the different gender or left-right side variables. The variables of the SWM and CPTs accompanied by changes in pain duration, intensity and features of patients with HON were investigated.
The Predicting Values for HON
Based on the clinical observations and pain scores, 155 subjects with HON (79 men and 76 women; mean age: 64.4±6.5 years; range: 50–75 years; 93 subjects affected on the right side; 153 subjects were primary school or higher educational level) were selected to measure their SWM and CPTs at pain sites. The results of ROC analysis for each of the SWM and CPTs are summarized in Figure 1 and Table 3.
Figure 1.Receiver operating characteristics curve of Semmes-Weinstein monofilament testing and current perception threshold testing at 3 frequencies for herpetic ophthalmic neuralgia.
| AUC(95% CI) | Sensitivity(%) | Specificity(%) | Cut-offvalue | Hyperesthesia< LRL (n) | Normal range(n) | Hypoesthesia>Cut-off (n) | Pain durationsp | Pain intensityp | Pain featuresp | |
| SWM (g) | 0.79(0.71 to 0.86) | 83 | 73 | 0.135 | 0.041 (2) | 0.042 to 0.135(25) | >0.136 (128) | 0.092 | 0.359 | 0.252 |
| CPT5Hz(mA×100) | 0.91(0.87 to 0.95) | 86 | 88 | 53 | 18 (8) | 18 to 53 (14) | >54 (133) | 0.833 | 0.832 | 0.095 |
| CPT 250Hz (mA×100) | 0.94(0.91 to 0.97) | 84 | 93 | 66 | 30 (1) | 30 to 59 (16) | >60 (138) | 0.359 | 0.725 | 0.000* |
| CPT2000Hz (mA×100) | 0.93(0.89 to 0.96) | 82 | 97 | 199 | 100 (6) | 101 to 199 (22) | >200 (127) | 0.427 | 0.475 | 0.002* |
The SWM and CPTs indicated significant predictive values for healthy controls compared to subjects with HON (AUC > 0.75). Among the 4 ROCs, CPT2000Hz was identified for predicating healthy controls to HON, and the AUC of the CPT2000Hz was 0.93 with both high sensitivity (81.9%) and specificity (96.7%), and the corresponding cut-off value was 199. In contrast, the SWM had lowest sensitivity (80.6%) and specificity (73.3%), and the corresponding cut-off value was 0.135.
Based on a normal distribution of the SWM and CPTs of healthy controls, the average values and the upper and lower normal reference limits (defined as +/- standard deviations from the mean) for each testing were calculated28. It showed that these cut-off values for the CPTs and SWM were approximately equal with the mean + standard deviation, which were defined as the upper reference limits, except for CPT2000Hz. Combined lower reference limits with the cut-off values were then used as a screening hyperesthesia or hypoesthesia threshold for local sensory malfunction of HON. Table 3 showed the hyperesthesia, normal or hypoesthesia distributions of 155 subjects with HON. More than eighty percent of the HON subjects had either SWM or CPT hypoesthesia at any frequency in the pain area. In contrast, only 0.64─5.16% of subjects with HON had either SWM or CPT hyperesthesia.
Variations in the SWM and CPTs with Pain in HON
One hundred fifty-five patients had a clinically manifested sensory impairment in the affected areas involving every sensory modality. Most of patients also had abnormal SWM and CPTs. The Shapiro-Wilk test for the SWM and CPTs of patients showed positively skewed distributions, and the median (IQR) of the SWM and CPTs were calculated. We used the PS/MS ratio of the SWM and CPTs to avoid the potential impact of individual differences on intra-individual comparisons.
According to the length of time pain lasted days after rash onset, subjects with HON were labeled HON-30, HON-60, HON-90, HON-120 and HON>120 days. The PS/MS ratio of the CPTs and SWM were compared among the different pain duration groups. A Kruskal-Wallis H test showed no significant differences in the SWM and CPTs among different pain duration groups.
The worst pain scores reported by subjects with HON ranged from 4 to 10 on the NRS (mean 7.3). The PS/MS ratio of the CPTs and SWM were compared among the different pain intensity groups. A Kruskal-Wallis H test showed no significant differences in the SWM and CPTs among the different pain intensity groups.
The PS/MS ratio of the CPTs and SWM were compared among the different pain characteristic groups, and significant differences in CPT values for 250Hz and 2,000Hz frequencies among the different groups were observed (shown in Figure 2, p<0.05). Post hoc tests showed significant difference in CPT250Hz between itching and lancinating pain (p=0.006), itching and allodynia (p =0.002), and burning pain and allodynia (p =0.042). Similarly, the CPT2000Hz between deep pain and allodynia (p =0.001) and itching and allodynia (p =0.014) were significantly different.
Figure 2.Comparison the ratio of pain site to the mirror site of Semmes-Weinstein monofilament testing and current perception threshold testing at 3 frequencies with the different pain characteristic groups. Semmes-Weinstein monofilament testing and current perception threshold testing values for each subject were shown, and the horizontal line indicated the median (IQR) scores for each group. * An overall comparison using the Kruskal-Wallis test indicated significant differences among groups (p< 0.05).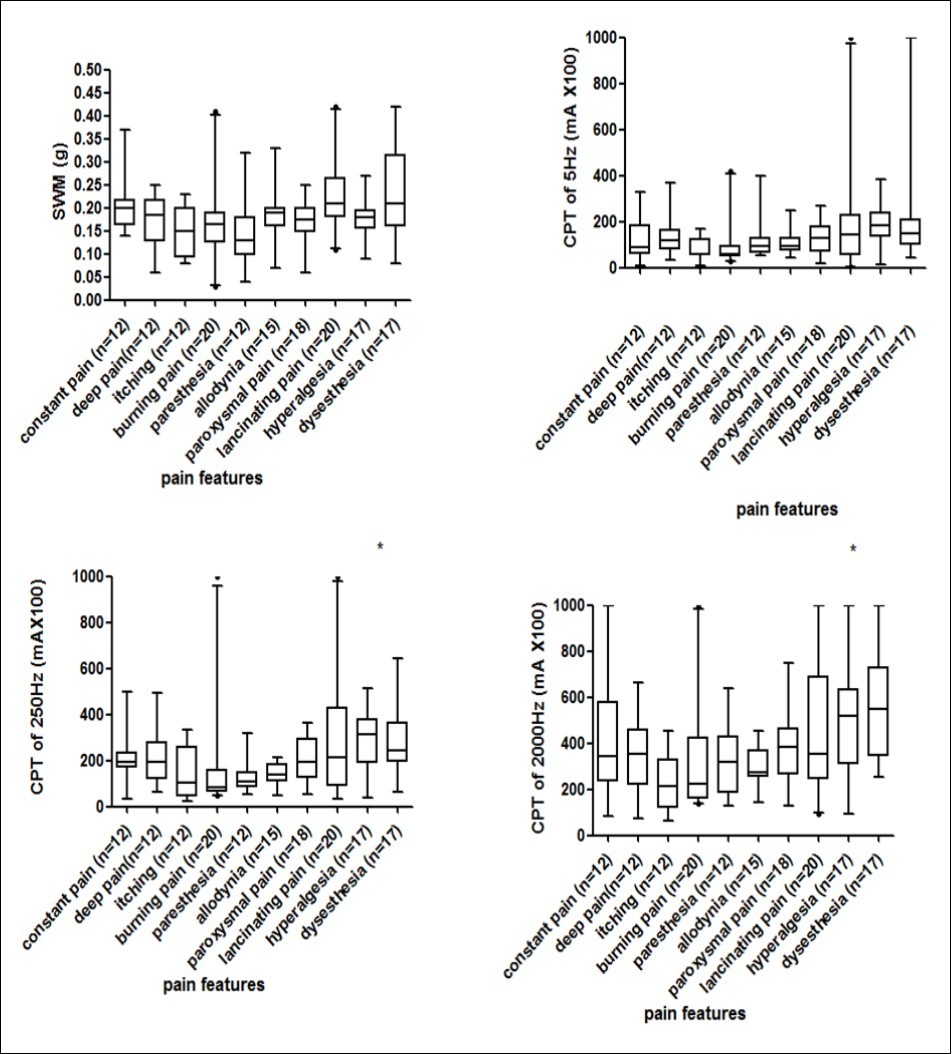
Discussion
There was a strong correlation between the incidence of HZ and increasing age, with a marked rise in incidence at the age of 50–60 years and older29. We thus selected the age 50 years as a point for comparison against the HZ and HON populations. Previous reports using a variety of stimulation techniques suggested differences in sensory thresholds between genders were related to differences in skin fold thickness and epidermal nerve fiber density30, 31. Our results suggested there were no significant differences when comparing the SWM and CPTs in the supraorbital region in men and women or the right and left sides.
HON in the V1 distribution has a complex pathophysiology that begins with viral damage and increased sensitization of peripheral sensory fibers 4, 32. The persistence of local pain after the onset of vesicles implies that the damaged V1 branch play an important role in the pathogenesis of herpetic neuralgia33. However, accurately ascertaining these conditions is difficult, and quantifying the severity of pain and sensory disturbance has generally been considered impossible. The development of new methods for assessing neuropathic pain has provided an improved foundation for the diagnosis and assessment of herpetic neuralgia. The SWM testing is one of the clinical tests that measures the response to a touching sensation of the monofilaments using a numerical quantity. This testing was developed for the detection of patients at risk of neuropathic ulceration, and it is a clinical tool used in the evaluation of peripheral nerve injuries and compression syndromes before and/or after recovery34, 35, 36, 37, 38, 39, 40, 41, 42. During the past three decades, the CPTs have been reported to provide reliable measures of peripheral sensory nerve function from the large and small myelinated and unmyelinated nerve fibers using a neurometer 43, 44. As the worst pain associated with HZ are the partial lesion regions innervated by distal nerve branches instead of the entire nerve distribution area [12], the local SWM and CPT changes may be reflected in the functions of supraorbital nerve fibers with HON.
Although it is difficult to prove the CPTs could selectively measure the functional integrity of the large myelinated, small myelinated and unmyelinated C-fibers, CPT evaluation has the unique ability to detect both hyperesthesia (abnormally low electrical excitation thresholds) as well as hypoesthesia (abnormally elevated thresholds)45. In the present study, we evaluated the SWM and CPT values in 155 patients with HON. Using the means and standard deviations from normative data and the cut-off values from ROCs, the SWM and CPTs provided high sensitivity and specificity for detecting the local neural function of HON. More than eighty percent of subjects with HON had either SWM or CPT hypoesthesia, and 0.64─5.16% subjects with HON had hyperesthesia at any frequency in the pain area. Assessment of the functions of these nerve fibers involved in acute and chronic pain, is clinically most challenging for patients with HON, thus contributing greatly to improved diagnosis and treatment. However, the results of SWM and CPT testing did not correlate with pain duration or pain intensity, which implied these lower reference limits and cut-off values had little relation to underlying mechanisms.
Most patients with HON describe multiple types of pain12. The characteristics of the pain and intensity were obviously due to different pathophysiological mechanisms. Thus, the diagnosis and evaluation of pain on the basis of local sensory abnormalities might be more appropriate. The present study showed that the PS/MS ratio of the CPTs and SWM, when comparing different pain characteristic groups, were significantly different among the groups in the CPT values when using 250Hz and 2,000Hz frequencies (p<0.05). Post hoc tests showed significant differences in CPT250Hz between itching and lancinating pain (p=0.006), itching and allodynia (p =0.002), and burning pain and allodynia (p =0.042). Additionally, there were significant differences in CPT2000Hz between deep pain and allodynia (p =0.001) and itching and allodynia (p =0.014). These results implied that CPT250Hz or CPT 2000Hz of allodynia were different from those of itching, burning pain or deep pain, which indicated that the neurometer can be used as an auxiliary diagnostic technique for allodynia. Our results are supported by the findings of Ohsawa46 and Raj47 et al. The results of the SWM had relatively low sensitivity to identify the pain characteristics.
The present study showed that combined SWM and CPT testing could be used to assess local sensory function with HON, which is a less invasive test that can be performed anywhere on the body in a simple manner. The test can be performed without any limitations in location, and it is a useful test for evaluating the local sensory fiber functional changes with HON.
Funding sources
This work was supported by the Shanghai Science and Technology Committee (16401934900) and Shanghai Municipal Commission of Health and Family Planning (20134320). The sponsorship was limited to supplies and expenses. The financial sponsor of this work had no role in the design and conduct of the study or the collection, management, analysis, interpretation of the data and preparation or review of the manuscript or the decision to submit. The remaining authors have no conflicts of interest.
Acknowledgements
The authors thank the patients who served as participants for this study, and Drs Wei-zhen Tang, BSc, Jie Xu, BSc, and Wen Li, BSc (Department of Rehabilitation Medicine, Shanghai Tenth People’s Hospital) for data collection.
References
- 1.Weaver B A. (2009) Herpes zoster overview: natural history and incidence. , J Am Osteopath Ass;109: 2-6.
- 2.Hope-Simpson R E. (1965) The Nature of Herpes Zoster: A Long-Term Study and a New Hypothesis. Proc R Soc Med. 58, 9-20.
- 3.Dunteman E. (2002) Peripheral nerve stimulation for unremitting ophthalmic postherpetic neuralgia. Neuromodulation :. , journal of the International Neuromodulation Society 5, 32-37.
- 4.Alvarez F K, de Siqueira SR, Okada M. (2007) Evaluation of the sensation in patients with trigeminal post-herpetic neuralgia. J Oral Pathol Med. 36, 347-350.
- 5.Liesegang T J. (2008) Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity.Ophthalmology2008;115:. 3-12.
- 7.Nithyanandam S, Dabir S, Stephen J. (2009) Eruption severity and characteristics in herpes zoster ophthalmicus: correlation with visual outcome, ocular complications, and postherpetic neuralgia. , Int J Dermatol 48, 484-487.
- 8.Gilden D, Nagel M A, Cohrs R J. (2013) The variegate neurological manifestations of varicella zoster virus infection. Current neurology and neuroscience reports. 13-374.
- 9.Bennett G J.Hypotheses on the pathogenesis of herpes zoster-associated pain. AnnNeurol1994;35Suppl: 38-41.
- 10.Johnson R, McElhaney J, Pedalino B.Prevention of herpes zoster and its painful and debilitating complications.IntJInfectDis2007;11Suppl2:S43-48.
- 11.Ragozzino M W, Melton L J, Kurland LT 3rd. (1982) Population-based study of herpes zoster and its sequelae. Medicine (Baltimore). 61, 310-316.
- 12.Dworkin R H, Gnann J W, Oaklander A L. (2008) Diagnosis and assessment of pain associated with herpes zoster and postherpetic neuralgia. , J Pain 9, 37-44.
- 13.Oaklander A L. (2001) The density of remaining nerve endings in human skin with and without postherpetic neuralgia after shingles. Pain. 92, 139-145.
- 14.Zaichick S V, Bohannon K P, Smith G A. (2011) Alphaherpesviruses and the cytoskeleton in neuronal infections. Viruses. 3, 941-981.
- 15.DaSilva A F, DosSantos M F. (2012) The role of sensory fiber demography in trigeminal and postherpetic neuralgias. , J Dent Res 91, 17-24.
- 16.Moharic M, Vidmar G, Burger H. (2012) Sensitivity and specificity of von Frey's hairs for the diagnosis of peripheral neuropathy in patients with type 2 diabetes mellitus. Journal of diabetes and its complications. 26, 319-322.
- 17.Rolke R, Baron R, Maier C.Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values.Pain2006;123:. 231-243.
- 18.Suzuki Y, Ogawa K, Shiota H.Current perception threshold in subacute myelo-optico-neuropathy. The International journal of neuroscience2010;120: 368-371.
- 19.Liao M F, Lee M, Hsieh M J. (2010) Evaluation of the pathophysiology of classical trigeminal neuralgia by blink reflex study and current perception threshold testing. The journal of headache and pain. 11, 241-246.
- 20.Griffith K A, Couture D J, Zhu S. (2014) Evaluation of chemotherapy-induced peripheral neuropathy using current perception threshold and clinical evaluations. Support Care Cancer. 22, 1161-1169.
- 21.Lee W C, Wu H C, Huang K H.Hyposensitivity of C-fiber afferents at the distal extremities as an indicator of early stages diabetic bladder dysfunction in type 2 diabetic women. PLoS One 2014: 9:. 86463.
- 22.Zheng W, He Y, Chen L. (2012) Correlation of current perception threshold and somatosensory evoked potential in diabetes. Neurophysiol Clin. 42, 241-247.
- 23.Sakai T, Tomiyasu S, Yamada H. (2006) Evaluation of allodynia and pain associated with postherpetic neuralgia using current perception threshold testing. , Clin J Pain 22, 359-362.
- 24.Truini A, Galeotti F, Haanpaa M.Pathophysiology of pain in postherpetic neuralgia: a clinical and neurophysiological study.Pain2008;140:. 405-410.
- 25.Voerman V F, J van Egmond, Crul B J. (2000) Elevated detection thresholds for mechanical stimuli in chronic pain patients: support for a central mechanism. Archives of physical medicine and rehabilitation. 81, 430-435.
- 26.Caissie R, Landry P E, Paquin R. (2007) Quantitative method to evaluate the functionality of the trigeminal nerve. , Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons 65, 2254-2259.
- 27.Wallace M S, Dyck J B, Rossi S S. (1996) Computer-controlled lidocaine infusion for the evaluation of neuropathic pain after peripheral nerve injury. Pain. 66, 69-77.
- 28.Horn P S, Pesce A J, Copeland B E. (1998) A robust approach to reference interval estimation and evaluation. Clinical chemistry. 44, 622-631.
- 29.Johnson R W, McElhaney J. (2009) Postherpetic neuralgia in the elderly. International journal of clinical practice. 63, 1386-1391.
- 30.Leitgeb N, Schroettner J, Cech R. (2005) Electric current perception of the general population including children and the elderly. , J Med Eng Technol 29, 215-218.
- 31.Maffiuletti N A, Herrero A J, Jubeau M. (2008) Differences in electrical stimulation thresholds between men and women. Ann Neurol. 63, 507-512.
- 32.Wood M.Understanding pain in herpes zoster: an essential for optimizingtreatment.JInfectDis2002;186Suppl1:S78-82.
- 33.Whitley R J, Volpi A, McKendrick M.Management of herpes zoster and post-herpetic neuralgia now and in the future. , J Clin Virol 2010: 48 Suppl 1, 20-28.
- 34.McGill M, Molyneaux L, Yue D K. (1998) Use of the Semmes-Weinstein 5.07/10 gram monofilament: the long and the short of it. Diabetic medicine : a journal of the British Diabetic Association. 15, 615-617.
- 35.Martinez-Hervas S, Mendez M M, Folgado J. (2017) Altered Semmes-Weinstein monofilament test results are associated with oxidative stress markers in type 2 diabetic subjects. , Journal of translational medicine 15-187.
- 36.Maracle E C, Hung L Y, Fell S I. (2017) A Comparison of the Sensitivity of Brush Allodynia and Semmes-Weinstein Monofilament Testing in the Detection of Allodynia Within Regions of Secondary Hyperalgesia in Humans. Pain practice : the official journal of World Institute of Pain. 17, 16-24.
- 37.Snyder B A, Munter A D, Houston M N. (2016) Interrater and intrarater reliability of the semmes-weinstein monofilament 4-2-1 stepping algorithm. Muscle & nerve. 53, 918-924.
- 38.Hire J M, Ramadorai U E, Contractor D.Intravenous angiocatheters as a novel alternative to Semmes-Weinstein monofilament evaluation in testing protective sensation.MilMed2014;179:. 442-444.
- 39.Slater R A, Koren S, Ramot Y. (2014) Interpreting the results of the Semmes-Weinstein monofilament test: accounting for false-positive answers in the international consensus on the diabetic foot protocol by a new model. Diabetes Metab Res Rev. 30, 77-80.
- 40.Slater R A, Koren S, Ramot Y. (2013) Pilot study on the significance of random intrasite placement of the Semmes-Weinstein monofilament. Diabetes Metab Res Rev. 29, 235-238.
- 41.Ellaway P H, Catley M. (2013) Reliability of the electrical perceptual threshold and Semmes-Weinstein monofilament tests of cutaneous sensibility. Spinal Cord. 51, 120-125.
- 42.Hsu H Y, Kuo L C, Chiu H Y. (2009) Functional sensibility assessment. Part II: Effects of sensory improvement on precise pinch force modulation after transverse carpal tunnel release. , J Orthop Res 27, 1534-1539.
- 43.Oishi M, Mochizuki Y, Suzuki Y. (2002) Current perception threshold and sympathetic skin response in diabetic and alcoholic polyneuropathies. Intern Med. 41, 819-822.
- 44.Katims J J. (1997) Neuroselective current perception threshold quantitative sensory test. Muscle & nerve. 20, 1468-1469.
- 45.Takekuma K, Ando F, Niino N. (2002) Prevalence of hyperesthesia detected by current perception threshold test in subjects with glucose metabolic impairments in a community. Intern Med. 41, 1124-1129.
