Persistent Neovascular Exudation in Patients with Exudative Age-Related Macular Degeneration who have Choroid Imaging Biomarkers of Non-Neovascular Choroidal Pathology: Simultaneous Choroidal Hyperpermeability and Angiogenesis
Abstract
Purpose
Create a new diagnostic and therapeutic framework for patients with Exudative Age-Related Macular Degeneration (ARMD) and choroid imaging biomarkers of non-neovascular choroidal pathology who have persistent neovascular exudation during the course of monotherapeutic interventions.
Methods
Retrospective, longitudinal case series study of 25 eyes from 23 patients with the referral diagnoses of treatment resistant Exudative ARMD who had persistent neovascular exudation despite various monotherapies. Inclusion criteria required choroidal imaging biomarkers of non-neovascular pathology including a thickened subfoveal choroid (greater than 300 microns) and vessels (subjectively dilated choroidal vessels in Haller’s layer) on Optical Coherent Tomography (OCT), choroidal neovascularization on IVFA and OCT Angiography (OCTA), as well choroidal leakage noted on indocynanine green videoangiography (ICG). Treatment consisted of OCTA and ICG - Directed Photodynamic Therapy (PDT) Triple Therapy, hereafter described as Combination Therapy, to areas of choroidal hyperpermeability and choroidal neovascularization. Combination therapy consisted of an anti-Vascular Endothelial Growth Factor (VEGF) intravitreal injection on Day 0 followed by half-fluence PDT and 2 mg intravitreal triamcinolone acetonide on Day 3-14.
Results
All study patients had treatment resistant Exudative ARMD defined as persistent subretinal and/or intraretinal fluid during their course of monotherapeutic interventions. Complete resolution of all exudation occurred in 23 eyes (92.0%) at 8 weeks. The mean duration of action was 155.6 weeks, with 72.0% of eyes leak free greater than 100 weeks. The mean vision at baseline was 0.46 ± 0.42 LogMAR, best corrected visual acuity (BCVA). 8 weeks after treatment, the vision was 0.35 ± 0.38 LogMar, an improvement of over one line, and this was maintained at one year. The baseline central subfield thickness (CST) was 296.4 ± 136.1 microns and improved by 111.4 ± 105.4 microns at 8 weeks after treatment. Treatment duration was negatively associated with the Caucasian race.
Conclusions
Patients with subretinal and/or intraretinal fluid secondary to Exudative ARMD should have a complete baseline multimodality imaging study to confirm the presence of neovascularization and whether choroidal hyperpermeability coexists. This study shows that patients with Exudative ARMD and persistent neovascular exudation despite monotherapuetic interventions often have choroidal biomarkers of non-neovascular choroidal pathology and that ICG and OCTA-directed PDT Triple Therapy resulted in complete resolution of all exudation in 92.0% of patients at 8 weeks with a reduction in central subfield thickness (CST) of 111.4 microns. The vision improvement at 8 weeks was 0.11 ± 0.38 LogMar and was sustained over 1 year. The mean duration of action was 155.6 weeks, with 72.0% of eyes leak free greater than 100 weeks. Additionally, this study shows that the treatment that addresses both pathological processes is successful and should be considered as a primary protocol when the biomarkers are present at baseline or as a secondary protocol if indeed the neovascular leakage is persistent despite monotherapy.
Summary
Patients with an Exudative ARMD with persistent neovascular exudation despite anti-VEGF monotherapy and who have imaging biomarkers of non-neovascular choroidal pathology often have two pathophysiological processes: choroidal hyperpermeability and angiogenesis. A proposed framework provides the rationale for OCTA and ICG-directed PDT Triple Therapy which successfully resolves 92% of the leakage that was persistent after various monotherapeutics.
Author Contributions
Copyright © 2025 Mark H. Nelson, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have no conflict of interest to declare.
Citation:
Introduction
The identification of selective angiogenic pathways mediated by Vascular Endothelial Growth Factor (VEGF) A, Placental Growth Factor (PlGF) and Angiopoietin 2 (Ang2) in patients with Exudative ARMD, represent an inflection point in the diagnosis and management of a family of blinding macular disease. Ferrara’s foundational research in 1989 1, as well as Folkman and other investigators in the field of oncology 2, revealed the importance of VEGFA in the neovascular and exudative process. Aiello 3showed its relationship to the pathophysiology of neovascular and exudative complications. and supported 4 the concept of blocking VEGF as a potential therapeutic target. The development of the first anti-VEGFA treatment, the monoclonal antibody bevacizumab, which received FDA approval for cancer treatment in 2004 5 followed by anti-VEGFA drugs for retinal disease, specifically pegaptanib 6, ranibizumab 7,8 and brolucizumab 9 , anti-VEGF and anti-PlGF drugs, specifically aflibercept10 , and the bispecific anti-VEGF and anti-Ang2 drug, specifically faricimab11 represented an important first step towards creating a personalized approach to treating neovascularization and subsequent exudation.
Although anti-VEGF therapy is extremely safe and effective, a wide variability of clinical response is common and has been summarized by Ting Yap, et al 12. Their metanalysis of fifty studies concerning the treatment of neovascular ARMD showed that the pooled prevalence of retinal fluid was 41.4% at one year and 47.4% at two years. Additionally, 17.6% of patients never achieved complete intraretinal and/or subretinal fluid resolution during the trials with an extreme levels of variability ranging from 4.1% with faricimab to 33.9% with bevacizumab.
Variability can be partially explained by the heterogeneity of the treatment population of patients with Exudative ARMD. Schmitz-Valckenberg13 highlighted that "determining the causes of a broadly defined multifactorial disease without attempting to further classify its clinical manifestations is a far too simplistic approach and has likely hampered progress in ARMD research." Kato14 described four phenotypes in patients with subretinal macular neovascularization: 1) drusen-associated neovascular ARMD (nARMD) composed of patients with soft drusen and type 1 or 2 choroidal neovascularization, 2) polypoidal choroidal vasculopathy (PCV), a phenotype that had polypoidal dilations of type 1 choroidal neovascularization, however, did not have soft drusen, 3) retinal angiomatous proliferation (RAP), a group composed of Type 3 choroidal neovascularization, and 4) pachychoroid choroidal vasculopathy (PNV), a group of patients without soft drusen who had biomarkers of an abnormal choroid, namely choroidal thickening and large vessels on OCT, peripheral RPE tracks on autofluorescence (AF), and choroidal leakage on ICG. He further shows that patients with PNV and PCV did not present with soft drusen, one of the critical criteria for the diagnosis of Age-Related Macular Degeneration 15 .This heterogeneity would appear to be material to classic landmark clinical studies on ARMD as their inclusion criteria allowed patients as young as 50 years old and, more importantly, did not require the presence of soft drusen. Additionally, the presence of varied phenotypes in a heterogenous study population is important as adjunctive PDT has been shown to be important to accomplish exudative and neovascular resolution in patients with PCV 16 and in patients with nARMD neovascularization17, both Type 1 and 2, when arteriolarized through the PDGF-mediated addition of small muscle and pericytes, the presence of which is well document in animal studies 18,19 .
This paper examines the importance of phenotypic segmentation of patients with Exudative Maculopathy and shows the importance of multimodality imaging biomarkers to completely identify the pathological processes that are present. This paper will show that non-neovascular pathologic choroidal imaging biomarkers when paired with persistent neovascular exudation following various monotherapuetic interventions are a signal that further evaluation with multimodality imaging is indicated and that treatment protocols that include suppressing choroidal hyperpermeability are important. This paper examines the fourth phenotype as outlined by Kato that includes patients without soft drusen and which was characterized by Pang20as pachychoroid neovasculopathy. The purpose of this paper is not to litigate the concept of pachychoroid which is quite controversial due to the myriad of variable definitions and unclear relationship to a spectrum of disease processes 21. The purpose of this paper is to show that non-neovascular choroidal pathology may be present concurrently with angiogenesis and must be recognized and subsequently treated.
This paper proposes a framework that segments patients with neovascular exudation and imaging biomarkers of non-neovascular choroidal pathology based on age and pathologic mechanism. Each eye might present as a different segment and, in addition, each eye might have simultaneous patterns of one or more segments.
Methods
This study is a single center, retrospective review of 23 patients conducted in the Department of Ophthalmology, Atrium Health Wake Forest Baptist, with the referral diagnosis of treatment failure Exudative ARMD reviewed during the years 2016 to 2021. Treatment failure is defined as persistent subretinal or intraretinal fluid four weeks after the last monotherapeutic intervention. Inclusion criteria included subretinal and/or intraretinal fluid on OCT, the absence of soft drusen, choroidal imaging biomarkers including a thickened choroid (greater than 300 microns) with pachyvessels (as defined subjectively as large choroid vessels), ICG hyperflourescence, IVFA leakage in a non-specific pattern even if there were areas of an acute smokestack pattern of leakage, and OCTA evidence of choroidal neovascularization. In addition, the patient needed to be over 45 but less than 75 years old, a visual acuity between 20/20 and 5/200, and an intraocular pressure (IOP) less than 25 mm Hg. Exclusion criteria include the presence of myopic, histoplasmosis, or traumatic choroidal degeneration, angioid streaks, previous vitrectomy, optic neuropathy, diabetic retinopathy (edema or neovascular), traction maculopathies, and allergies to IVFA, ICG, dilating agents, PDT, or anti- VEGF medications.
Study patients were examined for visual acuity, slit lamp examination and intraocular pressure (IOP). Multimodality imaging including Spectral Domain OCT, IVFA and ICG angiography (HRA+OCT, Spectralis, Heidelberg Engineering, Heidelberg, Germany) were used for analysis. OCTA (Heidelberg Engineering) was used to determine the presence of choroidal neovascularization.
The treatment protocol included the intravitreal injection of ranibizumab (Lucentis, Roche), 0.5 mg per 0.05 ml or bevacizumab (Avastin, Roche) in one patient, 1.25 mg/0.05ml on Day 0, followed by PDT with verteporfin (Visudyne, Bausch and Lomb) and triamcinolone acetonide (Triescence, 2 mg per 0.05 ml, Bausch and Lomb) 3-14 days later. PDT was applied as half-fluence, based on time of exposure, with verteporfin (Visudyne), at a dose of 6 mg/meter square, with an infusion over 10 minutes. 15 minutes after the start of the infusion. A laser light at 689nm was delivered at a power of 25 J/cm square with an intensity of 300 mW/cm square over 42 seconds to each target spot which was based on ICG hyperflourescence and/or OCTA evidence of CNV that corresponded to clinical exudation.
Patients were assessed at baseline and followed every 4 weeks for two visits, then every 8 weeks after combination therapy until week 52. Best corrected visual acuity measurements were converted to logarithm of the minimum angle of resolution (logMAR). In addition, IOP, OCT and OCTA were measured on each follow up exam. IVFA and ICG were performed in all patients at baseline and whenever recurrent leakage recurred.
The primary outcome of this study is the proportion of eyes with complete reabsorption of subretinal fluid (defined as treatment success) at 8 weeks after treatment. Secondary outcomes include change in central subfield thickness (CST), duration of treatment effect, change in visual acuity and IOP.
Informed Consent was not obtained in this retrospective review. Institutional Review Board approval was obtained. This research adhered to the tenets of the Declaration of Helsinki.
Analysis
Baseline characteristics and pre/post treatment measurements are presented as mean ± standard deviation for continuous variables and number (percent) for categorical variables. Changes in visual acuity, CST, and extrafoveal CST (nonCST) were assessed with a paired t-test. Those with severe choroidal hyperpermeability were compared to those with less than severe choroidal hyperpermeability using the Student’s t-test. Duration in weeks of treatment success per eye was modeled as a function of fixed effects for age, race/ethnicity, AF location, chronic CME, and OCTA, and a random effect for participant in a multivariable mixed effects regression model. Statistical significance was set at an alpha of 0.05.
Results
25 eyes from 23 patients referred with the diagnosis of treatment failure Exudative Age-Related Macular Degeneration were evaluated in this retrospective study. A thickened subfoveal choroid with an EDI greater than 300 microns as well as pachyvessels (subjectively dilated choroidal vessels in Haller’s layer) were found in all 25 eyes (100.%). The mean Enhanced-Depth Imaging (EDI) of baseline subfoveal measurements was 460.0 ± 140.3 microns. Choroidal hyperfluorescence due to choroidal hyperpermeability was identified on ICG imaging in all 25 eyes (100.0%). Choroidal neovascularization (CNV) was found in all 25 eyes (100.0%) of this patient population. All 25 of the eyes (100.0 %) had simultaneous pathological processes consisting of choroidal hyperpermeability and angiogenesis, present.
The mean vision at baseline was 0.43 ± 0.41 LogMAR best corrected visual acuity (BCVA). The mean baseline CST was 304.7 ± 126.9 microns. The auto-fluorescence imaging shows central discontinuity in 7 eyes (28.0%), peripheral changes in 13 (52.0%), and both in 5 (20.0%).
Treatment prior to referral included several monotherapies including reduced fluence PDT in 5 eyes (20.0%), anti-VEGF injection(s) in 19 eyes (76.0%), systemic mineralocorticoid antagonists in 4 eyes (16.0%), macular thermal laser in one eye (4.0%), and micropulse subthreshold laser in 5 eyes (20.0%). 10 eyes (40.0%) had multiple treatment, however at no time was a combination of treatments employed. The treatment consisted of OCTA and ICG-Directed PDT Triple Therapy as described previously. Following one application, subretinal exudation resolved completely in 23 of the eyes (92.0 %) after 4 weeks and was maintained at 8 weeks. BVCA improved one line (0.11 +/- 0.20 LogMar) after treatment and was maintained at one year. The CST improved by 111.0 ± 105.9 microns at 8 weeks. The time between treatments has lasted a mean of 155.6 ± 97.4 weeks. The duration was segmented (Figure 1) into a first tertile (less than 100 weeks) in 7 eyes (28.0%), a second tertile (between 100 and 200 weeks) in 9 eyes (36.0%), and a third tertile (greater than 200 weeks) in 9 eyes (36.0%). Duration of action as a continuous variable in patient specific (Table 1, Table 2) data shows a statistical relationship between long duration of action and non-white patients as well as a tendency in younger patients. At the eye level, those with longer duration of action had a more significant visual improvement, as well as peripheral autoflourescent changes. Predictors of duration of treatment using multivariable regression analysis at the eye level shows (Table 3) shows a tendency for white patients and those with chronic CME to have a shorter duration of treatment effect.
Figure 1.Histogram of duration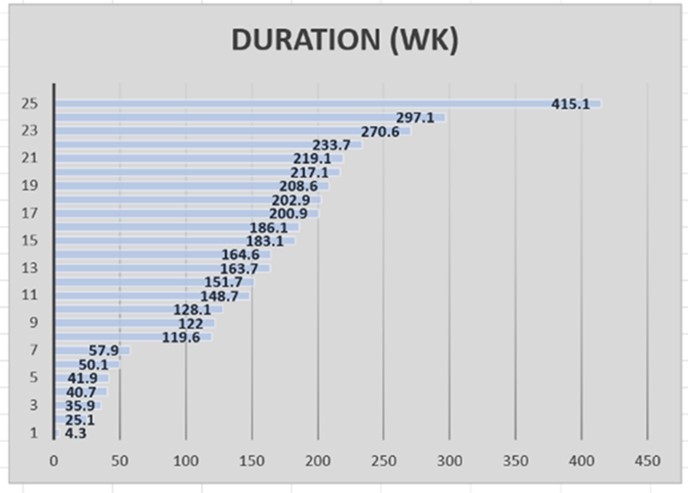
| Low duration (4.3-100 weeks)N=5 | Medium and Long duration (100-415.1 weeks)N=18 | Overall N=23 | P-value 1 | |
| Sex | 0.9274 | |||
| Male | 3 (60.0%) | 10 (55.6%) | 13 (43.5%) | |
| Female | 2 (40.0%) | 8 (44.4%) | 10 (56.5%) | |
| Age | 70.8 ±12.6 | 59.5 ±11.9 | 61.9 ±12.1 | 0.1607 |
| Age | 0.1896 | |||
| ≤ 62 | 1 (20.0%) | 11 (55.6%) | 12 (52.2%) | |
| > 62 | 4 (80.0%) | 7 (44.4%) | 11 (47.8%) | |
| Race/ethnicity | 0.0160 | |||
| White | 5 (100.0%) | 14 (77.8%) | 19 (82.6%) | |
| Non-White | 0 (0.0%) | 4 (22.2%) | 4 (17.4%) | |
| Cigarettes | 0.9748 | |||
| None | 2 (40.0%) | 13 (72.2%) | 15 (65.2%) | |
| Past/Current | 3 (60.0%) | 5 (27.8%) | 8 (34.7%) |
| Low duration (4.3-100 weeks) N=7 | Medium and Long duration (100-415.1weeks) N=18 | Overall N=25 | P-value | |
| Pre-VA | 0.4 ± 0.3 | 0.5 ± 0.4 | 0.4 ± 0.4 | 0.7469 |
| Change in VA | 0.05 ± 0.1 | 0.10 ± 0.2 | 0.10 ± 0.2 | 0.0306 |
| Steroid (J) | 0.7481 | |||
| None | 0 (0.0%) | 7 (38.9%) | 7 (28.0%) | |
| Nonoral/oral | 7 (100.0%) | 11 (61.1%) | 18 (72.0%) | |
| Imaging biomarkers | ||||
| EDI | 502.0 ±196.3 | 443.6 ±133.6 | 460.0 ±151.6 | 0.8164 |
| Pre-Treatment CST | 376.7 ±181.7 | 276.7 ± 90.0 | 304.7 ±126.9 | 0.7919 |
| Chronic CME | 0.8028 | |||
| 0 | 4 (57.1%) | 18 (100.0%) | 22 (88.0%) | |
| 1 | 3 (42.9%) | 1 (0.0%) | 3 (12.0%) | |
| PPP CNV | 0.9324 | |||
| 0 | 6 (85.7%) | 17 (94.4%) | 23 (92.0%) | |
| 1 | 1 (14.3%) | 1 (5.6%) | 2 (8.0%) | |
| RPED | 0.5937 | |||
| 0 | 7 (100.0%) | 16 (88.9%) | 23 (92.0%) | |
| 1 | 0 (0.0%) | 2 (11.1%) | 2 (8.0%) | |
| AF location (V) | ||||
| Central | 2 (28.6%) | 5 (27.8%) | 7 (28.0%) | |
| Peripheral | 4 (57.1%) | 9 (50.0%) | 13 (52.0%) | 0.07 |
| Both | 1 (14.3%) | 4 (22.2%) | 5 (20.0%) | 0.4857 |
| AF severity (W) | 0.1269 | |||
| Mild/Moderate | 5 (71.4%) | 17 (94.4%) | 22 (88.0%) | |
| Severe | 2 (28.6%) | 1 (5.6%) | 3 (12.0%) | |
| ICG | 0.9355 | |||
| 1/2 | 3 (42.9%) | 5 (27.8%) | 8 (32.0%) | |
| 3/4 | 4 (57.1%) | 13 (72.2%) | 17 (68.0%) | |
| OCTA | 0.9741 | |||
| None/1 | 2 (28.6%) | 10 (55.6%) | 12 (48.0%) | |
| 2/3 | 5 (71.4%) | 8 (44.4%) | 13 (52.0%) | |
| Group 1 | 0.436 | |||
| 0 | 2 (28.6%) | 2 (11.1%) | 4 (16.0%) | |
| 1 | 5 (71.4%) | 16 (88.9%) | 21 (84.0%) | |
| Group 2 | ---- | |||
| 0 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| 1 | 7 (100.0%) | 18 (100.0%) | 25 (100.0%) | |
| Group 3 | 0.7606 | |||
| 0 | 6 (85.7%) | 17 (94.4%) | 23 (92.0%) | |
| 1 | 1 (14.3%) | 1 (5.6%) | 2 (8.0%) | |
| Group Poly | 0.436 | |||
| 0 | 2 (28.6%) | 2 (11.1%) | 4 (16.0%) | |
| 1 | 5 (71.4%) | 16 (88.9%) | 21 (84.0%) | |
| 100% Resolve | 0.7696 | |||
| 0 | 2 (28.6%) | 0 (0.0%) | 2 (8.0%) | |
| 1 | 5 (71.4%) | 18 (100.0%) | 23 (92.0%) |
| Variable | Estimate (95% CI) | P-value |
|---|---|---|
| Age (years) | -0.5 (-4.4, 2.5) | 0.750 |
| Race/Ethnicity (ref: white) | ||
| Non-white | 93.5 (-7.5, 189.6) | 0.0818 |
| AF Location (ref: central) | ||
| Peripheral/both | -25.5 (-70.8, 28.9) | 0.576 |
| Chronic CME (ref: absent) | ||
| Present | -68.6 (-143.3, 15.5) | 0.228 |
| OCTA (ref: 0 or 1) | ||
| 2 or 3 | -27.9 (-21.8, 55.5) | 0.428 |
Discussion
This article outlines a unique framework (Table 4) that segments patients with macular subretinal exudation, neovascularization, and imaging biomarkers of non-neovascular choroidal pathology, based on pathophysiology as well as age. This framework leverages the importance of choroidal hyperpermeability and angiogenesis as simultaneous pathological processes and is critical to understanding the polymorphic nature of patient populations that we call Exudative ARMD.
Table 4. Pachychoroid/Exudative Maculopathy Framework| AGE | NAME | PRIMARY PATHOPHYSIOLOGY | |
| GROUP 1 | 25-45 | ACUTE CENTRAL SEROUS CHORIORETINOPATHY | CHOROIDAL HYPERPERMEABILITY |
| GROUP 2a | 45-75 | CHRONIC CENTRAL SEROUS CHORIORETINOPATHY | CHOROIDAL HYPERMEABILITY AND ANGIOGENESIS |
| GROUP 2b | 45-75 | PACHYCHOROID NEOVASCULOPATHY | ANGIOGENESIS |
| GROUP 3 | OVER 75 | EXUDATIVE AGE-RELATED MACULAR DEGENERATION | ANGIOGENESIS |
Group 1 is a cohort of young patients that have subretinal exudation, the biomarkers of choroidal pathology, however do not have neovascularization. Group 1 is included in this framework because patients with simultaneous angiogenesis and increased permeability found in Groups 2-4 often have signs of stigmata from previous disease, namely the peripheral RPE tracking and retinal atrophy consistent with previous choroidal hyperpermeability, which typically is from acute CSCR. The majority of these patients in Group 1 are 25-45 years old, are predominantly male, and are patients that have been diagnosed as Acute CSCR or Pachychoroid with Central Serous Retinopathy as described in the Pachychoroid Spectrum of Disease.21 Group 1 patients have serous detachments of the retina and RPE due to a pathognomonic focal leak in the Retinal Pigment Epithelium (RPE) as diagnosed on IVFA culminating in the “smokestack” late leakage pattern emanating from a finite number of leak sites 22. The OCT shows increased choroidal thickness and pachyvessels and the ICG imaging shows areas of hyperfluorescence that correspond to areas of OCT subretinal leakage.23 Varying degrees of RPE atrophy may be noted. The presence of peripheral involvement is pathognomonic for this cohort.24 Central RPE damage is typically secondary, i.e. the leakage migrates under the fovea from extramacular sources, and correlates well with vision loss. The absence of soft drusen and choroidal neovascularization is crucial to this Group assignment. The primary pathophysiological process is choroidal hyperpermeability.
Group 2 also have subretinal exudation, imaging biomarkers of non-neovascular choroidal pathology, however, in addition, have neovascularization. This cohort is older than Group 1, typically encompassing the ages of 45-75, has an equal male/female distribution, and has Type 1 choroidal neovascularization often requiring OCTA imaging for visualization where it manifests in a continuum from a “double layer” 25 sign to a prominent mature neovascular membrane, observed best with OCTA. The IVFA shows diffuse leakage consistent with the Type 1 occult neovascularization, and must be differentiated from the focal, pinpoint leaks found in Group 1. Autofluorescence often displays a central or peripheral disease process, unlike Group 1 which is always peripheral.
This group can be divided into two phenotypes. Group 2a have many of the characteristics of Group 1, i.e. choroidal hyperpermeability, however the exudation is chronic and CNV is present. Many of them have been referred as Exudative ARMD but share more characteristics of Acute CSCR, i.e. peripheral pigmentary tracks. Many of the Group 2a patients are consistent with the diagnosis of Chronic CSCR. Group 2b is similar to the Pachychoroid with Neovasculopathy cohort described within the Pachychoroid Spectrum of Disease framework.21 The process has submacular RPE changes and the absence of peripheral RPE changes as is seen in Group 2a. Despite the thick choroid and pachyvessels, ICG leakage is not prominent, however is present, therefore angiogenesis is the primary process. The patients in this study are all included in Group 2a and 2b.
Patients in Group 3 have the subretinal exudation, imaging biomarkers of choroidal pathology and choroidal neovascularization. They are typically over 75 and fall into the group that we call Exudative ARMD. This cohort has soft drusen and exudation that can be either intraretinal, subretinal or subRPE. Hemorrhage is not uncommon and neovascularization can be of any type. Like Group 2b, the process is central – the autofluorescence will show submacular changes. The IVFA is variable depending on the neovascular phenotype. The ICG may show varying degrees of hyperflourescence as well as arteriolarized neovascularization of Type 1 or 2 or a characteristic Type 3 Retinal Angiomatous Proliferation (RAP) lesions. Occasionally, peripheral tracks are present which probably indicate a Group 1 presentation earlier in life. The main pathological process in Group 3 is angiogenesis.
Group designation does not represent distinct phenotypes. It is hypothesized that they represent a continuum of macular exudative disease that starts in younger patients with choroidal hyperpermeability and ends in a disease process dominated by angiogenesis. Several Groups may be present simultaneously in one eye. For example, there may be peripheral tracks (Group 1) in an eye that has choroidal neovascular membrane present (Groups 2a or 3). This was suggested by Fung26who noted that his older cohort frequently had a history of Acute CSCR and that the development of choroidal neovascularization occurred a mean of 139 months after the former diagnosis (range 7-365 months). Other patients have a specific Group diagnosis(es) in one eye and another Group diagnosis, often asymptomatic, in the contralateral eye.
Figure 2.(A) right eye show autofluorescent discontinuity with peripheral tracks. (B) left eye show similar changes however central changes are present. (C), (D) OCT/EDI shows pachychoroid, pachy vessels, varying degrees of subretinal and intraretinal fluid, right eye (C), left eye (D). (E) IVFA right eye shows non-specific extrafoveal occult leakage. (F) ICG right eye with ICG hyperfluorescence and corresponding leakage. (G) IVFA leakage, left eye with several focal leaks amongst wider areas of non-focal leakage. (H) ICG shows diffuse hyperfluorescence corresponding to IVFA leakage. (I) OCT shows two images of the left macula with the “double layer sign” superior and a visible Type 2 neovascular membrane inferior, thickened choroid, and subretinal fluid and (J) with corresponding OCTA scans showing neovascularization.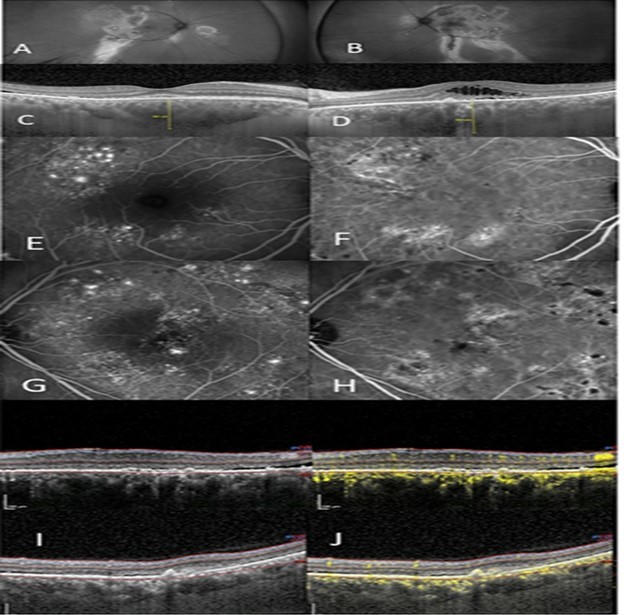
Figure 2 outlines a 52 year old Latino male, previously treated unsuccessfully with eplerenone (Inspra) and multiple anti-VEGF injections in both eyes. He was referred with the diagnosis of Exudative ARMD with a possible CSCR component due to his gender and age. He had chronic exudation, left eye greater than right eye, with macular atrophy left eye and subsequent vision loss. Autofluorescence of each eye reveal prominent peripheral discontinuity with classic gravitational tracks. Additionally, central changes are present in the left eye consistent with the macular atrophy and vision loss. IVFA of both eyes showed widespread extrafoveal exudation with areas of non-specific occult leakage as well as focal leaks. ICG hyperfluorescence shows diffuse choroidal leakage in both eyes. OCT showed a thickened choroid and pachyvessels with the OCTA revealing areas of “double-layer” sign. This patient had simultaneous Group 1 and Group 2a diagnoses.
Figure 3.(A),(B) autofluorescent discontinuity, peripheral, right eye, (A), left eye, (B). (C),(D) OCT shows pachychoroid, pachy vessels, intraretinal and subretinal fluid, right eye (C), left eye (D). (E) right eye IVFA with temporal occult and pinpoint leakage, (F) right eye ICG shows hyperfluorescence. (G) left eye shows nonspecific leakage nasally with corresponding ICG leakage (H). (I) shows OCTA with early CNV superior macula that is associated with the serous detachment of the macular.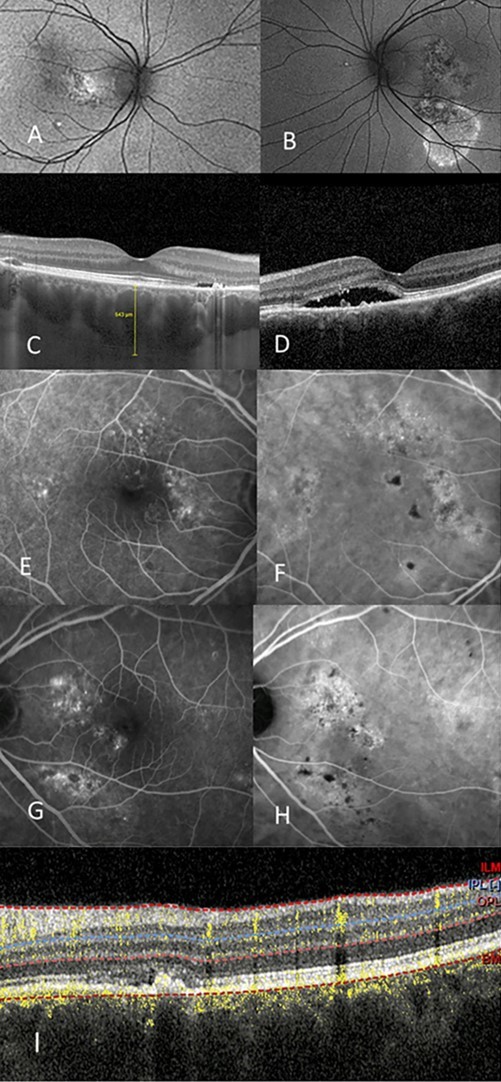
Figure 3 shows another patient with the referring diagnosis of Exudative ARMD with the possibility of CSCR due to her young age. She is a 52 year old Caucasian female who had been unsuccessfully treated with a series of single therapies: half fluence PDT, anti-VEGF intravitreal injections, and micropulse laser. Group 2a is the diagnosis in each eye. Figure 4 shows a 63 year old white male referred to as Exudative ARMD with a suggestion of Chronic CSCR due to his gender and age. This patient had a chronic serous detachment that did not respond to anti-VEGF monotherapy.
Figure 4.(A) Left shows autofluorescent changes, central and inferior, of the right eye. (B) Autofluorescent changes in the left eye which are much milder. (C) OCT right eye shows the thick choroid and serous detachment of the macula. (D) OCTA shows pachychoroid, double layer sign with neovascularization and serous detachment of the macula of the left eye. (E) IVFA of the right eye shows hypofluorescence from the subretinal fluid, however there are no leakage sites. (F) ICG of the right eye shows hyperfluorescence centrally and peripherally.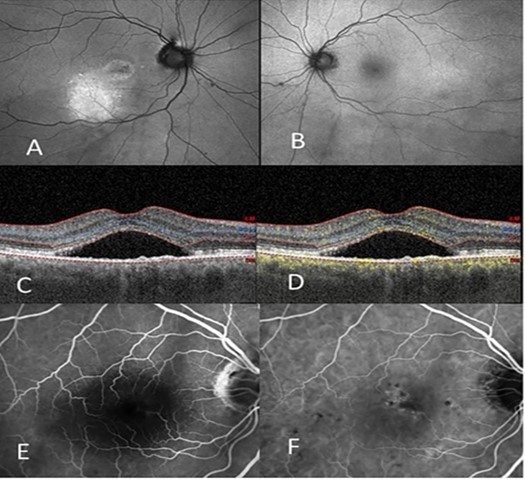
OCTA and ICG-directed PDT Triple Therapy was applied in all patients with complete resolution in 92% of the patients at 4 weeks then maintained at 8 weeks. The patients who did not respond had baseline chronic CME as noted in the OCT. Targeting of the PDT was always directed to areas of choroidal neovascularization (from OCTA) and ICG hyperfluorescence corresponding to the extent of the serous detachment (Figure 5).
Figure 5.(A) right eye OCT showing thick choroid, “double layer” sign, and serous detachment of the macula (B) right eye, s/p ICG and OCTA targeted PDT Triple Therapy (C) shows the IVFA targets (D) shows the ICG image revealing the two PDT targets with mapping of serous detachment (white arrow).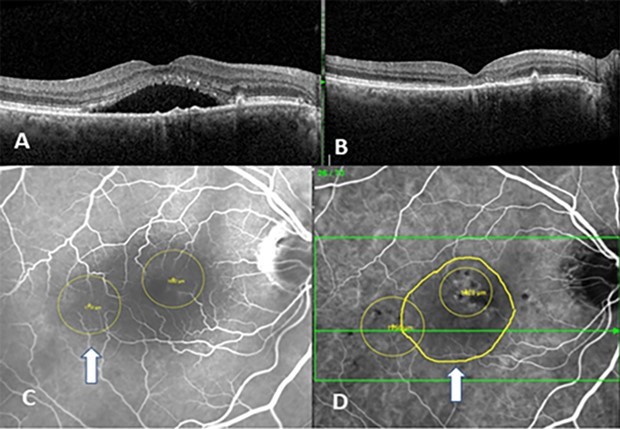
Patients with exudative maculopathy have historically been differentiated by age. Those who are younger, are given the diagnosis of Acute CSCR especially when pinpoint leakage is noted on IVFA, hyperflourescence on ICG, and there is an absence of soft drusen on OCT. Patients with Acute CSCR have historically been observed due to the likelihood of spontaneous resolution. Daruich 27 found that spontaneous resolution occurred in 83.9% of patients, with those with choroidal thickness over 500 microns, RPE elevation over 50 microns, and patient age over age 40 more likely to have suboptimal resolution.
Following the success of PDT after the TAP study for Exudative ARMD28 in 2000, PDT became the treatment of choice for Acute CSCR following a pilot study done by Lai, et al. 29 where half dose PDT with verteporfin was chosen to mitigate potential choroidal hypoperfusion and subsequent toxicity associated with full fluence dosing. The mechanism for PDT is directed towards occluding the hyperpermeable choriocapillaris endothelial cells via swelling, fragmentation, and detachment from its basement membrane with eventual occlusion without alterations to the RPE and retina.30 .Yannuzzi 31 outlined the use of ICG for targeted PDT to imaged areas of choroidal hyperpermeability in 2003. Anti-VEGF injections alone have not been found to be more effective than observation in the treatment of Acute CSCR32.
The diagnosis and treatment of Chronic CSCR is less clear. Daruich 33 attempted to clarify whether Chronic CSCR was merely the persistence of subretinal fluid past a certain time period, i.e. 4-6 months, with or without treatment, by segmenting patients with long standing Chronic CSCR based on history and clinical presentation and not just duration of exudation. She examined the ambiguity between exudation that ‘persisted’ greater than 4 months (non-resolving or “persistent” CSCR) as opposed to leakage that resolved and then recurred (“Recurrent” CSCR) due to new leakage sites. Lastly, she questioned the important question of whether Chronic CSCR is a different disease than Acute CSCR as opposed to a persistent exudation past a designated time period.
The question of the exact nature of Chronic CSCR and how it differs from Acute CSCR has been best characterized by Spaide 34 who noted that an older cohort had a higher degree of bilateral disease, an equalized male/female ratio, a larger percentage of IVFA leakage that was diffuse, and the clinical findings of a higher concentration of CNV. Fung 35 first showed the association between Chronic CSCR and Type 1 neovascularization with a study of 22 patients with Type 1 choroidal neovascularization who had characteristics more indicative of Chronic CSCR as opposed to Exudative ARMD. Savastano et al. 36 utilizing OCTA, reviewed 175 eyes with Acute and Chronic CSCR. No CNV were found in patients with Acute CSCR, however, CNV was found in 39.2% of all patient with Chronic CSCR and were all Type 1. Pang 20 described three patients with Type 1 neovascularization without evidence of previous central serous chorioretinopathy findings. She named this cohort Pachychoroid with Neovasculopathy and placed them into the Pachychoroid Spectrum of Disease framework. These patients are similar to the patients in the presented framework described in Group 2b. Chronic CSCR is more likely than Acute CSCR to have submacular RPE atrophy and photoreceptor deterioration,37 and cystoid deterioration 38
Photodynamic therapy for Chronic CSCR has been studied by randomized clinical trials which showed half-fluence PDT’s anatomic superiority over ranibizumab with complete exudative resolution occurring after one treatment (75% at three months in the pilot study and 88.9% in the second study).39 The PLACE study had a randomized arm treated with\ Half-Dose PDT which resulted in 67.2% of patients with complete exudative resolution.40 Haga looked at three year response data in patients with Chronic CSCR treated with half-fluence PDT 41. 81% of patients had sustained exudative resolution. Van Rijssen 42 noted that successful PDT responses were more likely if the patient was female, younger, had intense ICG hyper-fluorescence, better baseline visual acuity, and had focal leakage on IVFA as opposed to diffuse nonspecific leakage.
The concept of using anti-VEGF in Chronic CSCR was first described by Hage43. Bae 39 compared the use of ranibizumab versus half-fluence PDT in a randomized controlled study. 88.9% of eyes treated with PDT had complete resolution of exudation compared to 31.1% of the anti-VEGF treated group after the first treatment.
Micropulse laser for Chronic (CSCR) was first proposed by Bandelo.44 The PLACE study had a High Density Subthreshold Micropulse Laser (HSML) treatment arm 40 with complete resolution of exudation of 28.8% at 8 weeks. PLACE Report #3 segmented patients with Chronic CSCR into those who had fluorescein leakage that was either focal or diffuse 45, the former with a 38% response to HSML at 8 months compared to the latter where the resolution of leakage was 21%.
Bousquet 46 proposed a pilot study to use eplerenone, a mineralocorticoid receptor antagonist, to treat Chronic CSCR and found that 40.5% of patients had complete exudative resolution at 6 months when treated with eplerenone or spironolactone. Kim 47 showed a 69% resolution of exudation with spironolactone.
This paper illustrates that all ‘older patients’ with exudative maculopathy and who are found to have neovascular exudation and imaging biomarkers of non-neovascular choroidal pathology should not be automatically diagnosed as having Exudative ARMD especially when soft drusen are absent. Careful evaluation to determine whether treatment for choroidal hyperpermeability is mandatory for successful exudative resolution. If ICG hyperflourescence is present, PDT or anti-metabolite antagonist therapy will be necessary to treat the exudation. Additionally, if neovascularization is present, either as a “double layer” 25 sign or frank neovascularization on OCTA, the patient will require anti-VEGF treatment as well. If both are present, then the patient will need combination therapy as described above. This paper also illustrates that those with the diagnosis of Chronic CSCR should have a careful examination for the presence of CNV which may only be found on OCTA.
OCTA and ICG-directed PDT with verteporfin has been used both as a primary and rescue therapy for Exudative ARMD in patients with mature/arteriolarized neovascularization17 or polypoidal dilatation 16 of the neovascular tissue.
This study creates a hypothesis that the failure of monotherapy in patients with neovascular exudation and choroid imaging biomarkers of non-neovascular choroidal pathology in the absence of drusen whether they have been diagnosed as Exudative ARMD or Chronic CSCR, occurs when there is a failure to recognize that both choroidal hyperpermeability and angiogenesis are occurring simultaneously. This paper offers an important insight for Exudative ARMD clinical trials that use the lower age limit at 50 and do not require soft drusen to be included in their protocol criteria. It is likely that there might be patients with choroidal hyperpermeability in the study population.
Dariuich is likely correct in her hypothesis that Chronic CSCR is a different disease than Acute CSCR. I believe that there very well might be a continuum over years and that ARMD risk genes present in different phenotypes depending on age of the patient and the duration of the disease process.
Funding
None
References
- 1.Ferrara N. (1992) . Molecular and Biological Properties of VEGF Family of Proteins”. Endocrine Reviews 13(1), 18-32.
- 3.Aiello L. (1995) Vascular Endothelial Growth Factor. in Ocular Fluid of Patients with Diabetic Retinopathy and Other Retinal Disorders”. NEJM 331-1480.
- 4.Aiello L. (1995) Suppression of Retinal Neovascularization in vivo by Inhibition of VEGF using Soluble VEGF-Receptor Chimeric Proteins”. , PNAS 92, 10457-61.
- 6.Evangelos S. (2004) Pegaptanib for Neovascular Age-Related Macular Degeneration”. , N Engl J Med 351-2805.
- 7.Brown D. (2006) Ranibizumab versus Verteporfin for Neovascular Age-Related Macular Degeneration”. , NEJM 355-1432.
- 8.Rosenfeld P. (2006) . Ranibizumab in Patients with Subfoveal Neovascular Age-Related Macular Degeneration”. NEJM 355-1419.
- 9.Dugel P. (2020) 3, Multicenter, Randomized, Double-Masked Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration”. Ophthalmology. 127(1), 72-84.
- 11.Heier J. (2022) Durability, and Safety of Intravitreal Faricimab up to every 16 weeks for Neovascular. ARMD (TENAYA and LUCERNE):Two Randomized, Double-Masked, Phase 3, Non-inferiority Trials”. Lancet 399-729.
- 12.Yap Ting, D. (2025) Persistence of Retinal Fluid after Anti-VEGF Treatment for Neovascular ARMD: A Systematic Review and Meta-Analysis”. Ophthalmology Retina. 1-15.
- 13.Schmitz-Valkenberg S. (2022) From Genes, Proteins, and Clinical Manifestations: Why Do We Need to Better Understand Age-Related Macular Degeneration”? Ophthalmology Science. 2(2), 1-3.
- 14.Kato Y. (2022) . Age-Related Maculopathy Susceptibility 2 and Complement Factor H Polymorphism and Intraocular Complement Activation in Neovascular ARMD”. Ophthalmology Science 2, 1000167.
- 15.Curcio C. (2018) Antecedents of Soft Drusen, the Specific Deposits of Age-Related Macular Degeneration. in the Biology of Human Macula”. Invest Ophth Vis Sci 59, 182-190.
- 16.Koh A. (2012) Efficacy and Safety of Verteporfin Photodynamic Therapy. in Combination with Ranibizumab or Alone Versus Ranibizumab Monotherapy in Patients with Symptomatic Macular Polypoidal Choroidal Vasculopathy”. Retina 32(8), 1453-64.
- 17.M H Nelson. (2012) Primary ICG-Directed PDT Triple Therapy versus Standard of Care for the Treatment of Exudative Age-Related Macular Degeneration”. , ASRS Lecture
- 18.Benjamin L. (1998) A Plasticity Window for Blood Vessel Remodeling is Defined by Pericyte Coverage of the Preformed Endothelial Network and is Regulated by PDGF-B. 125-1591.
- 19.Jo N. (2006) Inhibition of Platelet-Derived Growth Factor B Signaling Enhances the Efficacy of Anti-Vascular Endothelial Growth Factor Therapy in Multiple Models of Ocular Neovascularization”. , American Journal of Pathology 168-6.
- 21.Siedlecki J. (2019) The Pachychoroid Disease Spectrum and the Need for a Uniform Classification System”. Ophthalmology Retina. 3, 1013-1015.
- 22.Yamada K. (1992) . Fluorescein-Angiographic Patterns in Patients with Central Serous Chorioretinopathy at the Initial Visit”. Ophthalmologica 205-69.
- 23.D R Guyer. (1994) Digital Indocyanine Green Videoangiography of Central Serous Chorioretinopathy”. Archives of Ophthalmology. 112(8), 1057-62.
- 24.A von Rückmann. (2022) Abnormalities of Fundus Autofluorescence in Central Serous Retinopathy. , Am J Ophthalmol 133-780.
- 25.Sato T. (2007) Tomographic Features of Branching Vascular Networks. in Polypoidal Choroidal Vasculopathy”. Retina 27-589.
- 26.A T Fung. (2012) . Type 1 (Sub-retinal Pigment Epithelial ) Neovascularization in Central Serous Chorioretinopathy Masquerading as Neovascular Age-Related Macular Degeneration”. Retina 32-1829.
- 27.Daruich A. (2017) Acute Central Serous Chorioretinopathy. Factors Influencing Episode Duration”. Retina. 37, 1905-1915.
- 28. (2001) Treatment of Age-Related Macular Degeneration With Photodynamic Therapy(TAP) Study Group. Photodynamic Therapy of Subfoveal Choroidal Neovascularization in Age-Related Macular Degeneration With Verteporfin: Two-Year Results of 2 Randomized Clinical Trials—TAP Report 2”. Arch Ophthalmol. 119-198.
- 29.Lai T Y Y. (2006) Safety Enhanced Photodynamic Therapy with Half Dose Verteporfin for Chronic Central Serous Chorioretinopathy: A Short Term Pilot Study”. Br. 90-869.
- 30.Schmidt-Erfurth U. (2002) Photodynamic Effects on Choroidal Neovascularization and Physiological Choroid”. , Invest. Ophthalmol. Vis. Sci 43-830.
- 31.L A Yannuzzi. (2003) Indocyanine Green Angiography-Guided Photodynamic Therapy for Treatment of Chronic Central Serous Chorioretinopathy: A Pilot Study”. , Retina 23, 288-296.
- 32.Aydin E. (2012) The Efficacy of Intravitreal Bevacizumab for Acute Central Serous Chorioretinopathy”. , Journal of Ocular Pharmacology and Therapeutics 29-10.
- 33.Daruich A. (2015) Central Serous Chorioretinopathy: Recent Findings and New Physiopathology Hypothesis”. Progress in Retinal and Eye Research 48, 82-118.
- 34.R F Spaide. (1996) . Central Serous Chorioretinopathy in Younger and Older Adults”. Ophthalmology 103-2070.
- 35.A T Fung. (2012) Type 1 (Sub-retinal Pigment Epithelial ) Neovascularization in Central Serous Chorioretinopathy Masquerading as Neovascular Age-Related Macular Degeneration. Retina. 32-1829.
- 36.M C Savastano. (2021) The Incidence of Neovascularization in Central Serous Chorioretinopathy by Optical Coherence Tomography Angiopathy”. Retina. 41, 302-308.
- 37.Wang M S M. (2002) Retinal Atrophy in Idiopathic Central Serous Chorioretinopathy”. , Am J Ophthalmol 133-787.
- 38.F C Piccolino. (2008) Posterior Cystoid Retinal Degeneration in Central Serous Chorioretinopathy. Retina. 28-1008.
- 39.S H Bae. (2014) Low-Fluence Photodynamic Therapy Versus Ranibizumab for Chronic Central Serous Chorioretinopathy: One-Year Results of a Randomized Trial”. Ophthalmology. 121-558.
- 40.Dijk E H C van. (2018) Half-Dose Photodynamic Therapy versus High-Density Subthreshold Micropulse Laser Treatment. in Patients with Chronic Central Serous Chorioretinopathy: The PLACE Trial”. Ophthalmology 125-1547.
- 41.Haga F. (2017) Long-Term Prognostic Factors of Chronic Central Serous Chorioretinopathy after Half-Dose Photodynamic Therapy: A 3-Year Follow-Up Study”. PLOS ONE. 12-0181479.
- 42.Rijssen T J van. (2018) Clinical Characteristics of Chronic Central Serous Chorioretinopathy Patients with Insufficient Response to Reduced-Settings Photodynamic Therapy”. Graefes Arch Clin Exp Ophthalmol. 256-1395.
- 43.Hage R. (2015) Flat Irregular Retinal Pigment Epithelium Detachments in Chronic Central Serous Chorioretinopathy and Choroidal Neovascularization”. , Am J Ophthalmol 159-890.
- 44.Bandello F. (2003) Non Visible Subthreshold Micropulse Diode Laser Treatment of Idiopathic Central Serous Chorioretinopathy. , A Pilot Study”. Invest. Ophthalmol. Vis. Sci 44, 4858-4858.
- 45.Rijssen T J van. (2019) Focal and Diffuse Chronic Central Serous Chorioretinopathy Treated With Half-Dose Photodynamic Therapy or Subthreshold Micropulse Laser:. PLACE Trial Report No. 3”. Am 205-1.
