Impact of Low Birth Weight on Early Vascular Aging and Cardiometabolic Phenotypes in Later Life Among Cameroonian Adults
Abstract
Background
Evidence suggests that low birth weight (LBW) is associated with increased cardiovascular and metabolic risk in adulthood, including increased arterial stiffness, a marker of early vascular aging (EVA) assessable by pulse wave velocity (PWV), obesity and glucose homeostasis abnormalities. The present study aimed to explore the late impact of LBW on PWV and cardiometabolic phenotypes among young adult Cameroonians.
Methods
The study evaluated 120 subjects (mean age: 26 ± 5 years; 54% male sex) at the Cameroon Heart Institute, Douala, Cameroon, between January and June 2018. Birth weight (BW) and gestational age, sociodemographic, anthropometrics and fasting capillary blood glucose were recorded in all participants. Blood pressure (BP) and PWV were measured using an automatic oscillometric device (Mobil-O-Graph®). Multiple-adjusted linear regression was used to determine predictive factors for PWV. For assessment of potential impact of BW on EVA, PWV was adjusted for age, sex, body mass index (BMI) and mean arterial pressure (MAP).
Results
28 participants (23.3%) of the study sample had LBW (<3000g). There was no gender difference between LBW or normal birth weight patients (NBW; controls). Age- and MAP-adjusted PWV (aPWV) were higher in women with LBW compared to NBW (5.6 m/s and 5.3 m/s respectively, P = 0.038). In men, aPWV was similar in LBW and NBW. In this study population, aPWV was higher (on average +15 cm/s) in LBW than in controls, although the difference was not statistically significant (P=0.083). Multivariate regression analysis showed age, male sex, BMI and MAP were independent determinants of PWV, but not LBW. Compared to NBW controls, the prevalence of overweight/obesity, impaired glucose homeostasis and diabetes was higher in LBW: 42.9% vs 37%; 10.7% vs 3.3%, and 3.6 % vs 1.1%, respectively. Moreover, compared with controls, LBW individuals who were overweight/obese in adulthood had a much higher mean fasting capillary glucose (1.54 ±0.17 g/l vs 0.87 ±0.11 g/l in NBW, p=0.003).
Conclusion
This study suggests that although LBW is associated with increased aortic stiffness in young adulthood, mainly in women, the association was predominantly driven by aging, MAP, BMI and male sex. In adulthood, LBW subjects exhibited higher obesity indices and altered glucose homeostasis.
Author Contributions
Academic Editor: Kavitha Menon, Public Health Foundation of India| Indian Institute of Public Health Gandhinagar (IIPHG) Sardar Patel Institute Campus, Drive-In Road, Thaltej, Ahmedabad- 380 054, Gujarat, India
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2020 Daniel Lemogoum, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have no conflicts of interest to declare.
Citation:
Introduction
Growing evidence suggests that low birth weight (LBW) adjusted for gestational age could adversely influence arterial stiffness, a marker of early vascular aging (EVA) measured by aortic pulse wave velocity (PWV), and blood pressure (BP) regulation 1, 2, 3, 4, 5, thereby increasing risk of cardiovascular (CV) events in adulthood 3, 4, 5, 6, 7, 8, 9, 10, 11. Indeed, an inverse relationship between birth weight (BW) and BP has been demonstrated, with higher risk of hypertension in adulthood 1, 2, 6, 7, 8, 9, 10. This association could be related either to renal organogenesis abnormalityor to decreased elastin synthesis within large arteries’ wall during fetal life 6, 12, 13, 14. In individuals with LBW, early remodeling of large arteries, such as the aorta, might occur during adult life, with increase in caliber and wall remodeling, with a decrease in elastic fibers content and an increase in collagen fibers in the media. This remodeling of large arteries’ wall was attributed to mechanical effects and growth factors, as well as cytokines and/or proteases 2, 12, 13, 14. Several studies have shown that the loss of elasticity in large arteries, leading to EVA, the latter characterized by raised arterial stiffness as measured by aortic pulse wave velocity (PWV), is an independent predictor of CV risk and overall mortality 14, 15, 16, 17, 18, 19, 20. It was also observed that individuals with fetal malnutrition and LBW were at increased cardiometabolic risk in adulthood, including incident type 2 diabetes mellitus (T2DM), obesity, and metabolic syndrome 21, 22, 23.
In Cameroon, despite a large number of subjects with a history of LBW, mostly caused by foetal growth retardation and materno-fetal malnutrition during high-risk pregnancies 24, to the best of our knowledge, no study has explored the late effects of LBW on EVA and cardiometabolic phenotypes in Cameroonian adolescents and adults. The present study was aimed to address this issue.
Methods
This was a cross-sectional study performed at the Cameroon Heart Institute, based in Douala, Cameroon between November 2017 and June 2018. We consecutively recruited apparently healthy Cameroonian individuals aged 15 to 30 years old, all non-smokers, in whom full data on BW and gestational age at birth were documented in the register of births. Individuals were excluded if they were taking medications or had any medical conditions that may have affected growth, maturation, physical activity, nutritional status, or their cardiometabolic health. Subjects with missing information for any of the variables were also excluded. All participants completed a validated questionnaire on personal and familial histories of CV or metabolic diseases, socio-demographics, and lifestyle habits. Anthropometric, hemodynamic and biological parameters collected included height, weight, waist circumference, BP, heart rate (HR), PWV, and fasting capillary blood glucose.
Hemodynamic Parameters
All hemodynamic measurements were performed, after a 15 minutes rest, in the sitting position and in standardized conditions 25. BP and PWV were recorded on the dominant arm. Systolic BP (SBP), diastolic BP (DBP), mean arterial pressure (MAP) calculed as DBP plus 1/3(SBP-DBP), HR, and PWV were measured using a validated brachial cuff-based oscillometric device (Mobil-O-Graph®; IEM; Stolberg, Germany). Two consecutive measurements were taken at time intervals of 5 minutes, using a cuff’s width adjusted to the arm’s circumference. In case of inaccurate recording, the measurement was repeated. The mean of two accurate hemodynamic readings was used. The device used was approved by the Food and Drug Administration, with its BP detection unit validated according to the European Society of Hypertension 26 recommendations. All procedures were performed by a single medical investigator.
Anthropometric and Biological Measurements
Height (m) was measured using a height gauge graduated in cm (Seca, Germany) and weight (kg) was measured using an electronic scale (Seca, Germany). The body mass index (BMI) was determined by dividing the weight (kilogram) by the square of the height (meter). Overweight was considered for a BMI ≥ 25 and <30.0 kg/m² and obesity for a BMI ≥30 kg/m². Waist circumference (cm) was measured, and abdominal obesity was considered for a waist circumference ≥102 cm (men) and ≥88 cm (women) 27. LBW for gestational age was defined BW adjusted for gestational age was below the 10th percentile or < -2 on the standard deviations from mean 1, 28.
Participants were instructed to fast for at least 8h overnight and fasting capillary blood glucose was determined using a glucometer (Accu-Chek Aviva, Roche, Mannheim, Germany). Diabetes mellitus was defined by a fasting capillary blood glucose ≥126 mg/dl, and impaired fasting glucose (“prediabetes”) defined as a fasting blood glucose between 100-125 mg/dl, in the absence of self-reported diabetes 29.
Ethical Considerations
Participation to the study was voluntary, and each participant gave prior informed consent. The confidentiality of data was ensured by anonymity of patients in data sheets. We obtained an ethical clearance from the Institutional Ethics Committee of the University of Douala (N°1259 CEI-Udo/02/2018/T).
Statistical Analyses
Quantitative data are presented as mean ± standard deviation while qualitative data are presented as count and percentages. Non-parametric Mann-Whitney and Chi2 tests have been used to compare quantitative and quantitative data respectively according to BW ranges. Multivariable linear regression model has been used to determine predictive factors for PWV. For assessment of potential impact of BW on EVA, PWV was adjusted for age, sex, MAP and BMI. Differences have been considered significant for p<0.05. All statistical analysis was performed using SPSS 20 software.
Results
Anthropometric, Hemodynamic and Biological Characteristics
The mean gestational age at delivery for our study participants was 39 weeks (Ranging from from 37 to 41 weeks) and the mean birth weight was 3267 g. Among the 120 participants, 28 (23.3%) had a history of LBW. Mean age was comparable between subjects with LBW and those with normal birth weight (NBW). The sex ratio was also similar between LBW and NBW groups (Table 1).
Table 1. characteristics of study participants according to birth weight| Total(N=120) | BW < 3000g(N=28) | BW≥3000g(N=92) | p | |
| Age (years) | 26 ±5 | 27±6 | 25±5 | 0.084 |
| Female | 55 (45.8) | 12 (42.9) | 43 (46.7) | 0.885 |
| BW (g) | 3267 ±560 | 2496±410 | 3501±354 | < 0.0001 |
| Weight (kg) | 72.4 ±13.9 | 72.1±14 | 72.5±14.1 | 0.68 |
| Height (m) | 1.71 ±0.09 | 1.70±0.09 | 1.71±0.09 | 0.601 |
| BMI (kg/m²) | 24.8 ±4.8 | 24.9±4.3 | 24.7±5 | 0.814 |
| Waist (cm) | 81 ±13 | 81.3±11.4 | 80.6±13 | 0.818 |
| Glycemia (mg/dl) | 99 ±8.9 | 114±13.1 | 94±7.3 | 0.105 |
| aPWV (m/s) | 5.45 ±0.34 | 5.52 ±0.39 | 5.37±0.27 | 0.083 |
| SBP (mmHg) | 128 ±2 | 129.0±2.6 | 128.0±2.1 | 0.084 |
| DBP (mmHg) | 76 ±5 | 78.1±5.9 | 75.8±4.6 | 0.084 |
| MAP (mmHg) | 100 ±4 | 101.1±4.2 | 99.4±3.3 | 0.084 |
| HR (bpm) | 74 ±10 | 74±9 | 74±10 | 0.557 |
Compared to NBW, the age- and MAP-adjusted PWV (aPWV) of LBW individuals in general was higher (on average +15 cm/s), although the observed difference did not reach statistical significance (P=0.089). The age adjusted SBP, DBP, and MAP levels were slightly higher, albeit non-significantly in subjects with LBW compared to NBW (p=0.084). Mean blood glucose was higher and in the pre-diabetes thresholds in those with LBW compared to subjects with NBW (P=0.105).
Association Between PWV and Age or MAP
As expected, the PWV was proportionally and positively correlated with chronological age (r=0.35; p<0.0001), while the increase in MAP resulted in a proportional acceleration of PWV (r=0.58; p<0.0001).
Distribution of CV Risk Factors as a Function of BW As shown in Figure 1, the prevalence of overweight/obesity, visceral obesity, prediabetes and diabetes was higher in individuals with LBW: 42.9% vs 37%, 35.7% vs 31.5%, 10.7% vs 3.3% and 3.6% vs 1.1% in individuals with NBW, respectively. By contrast, the age-adjusted prevalence of hypertension was higher in NBW subjects compared to those with LBW: 25 vs 21%.
Figure 1.Prevalence of cardiometabolic risk factors according to birth weight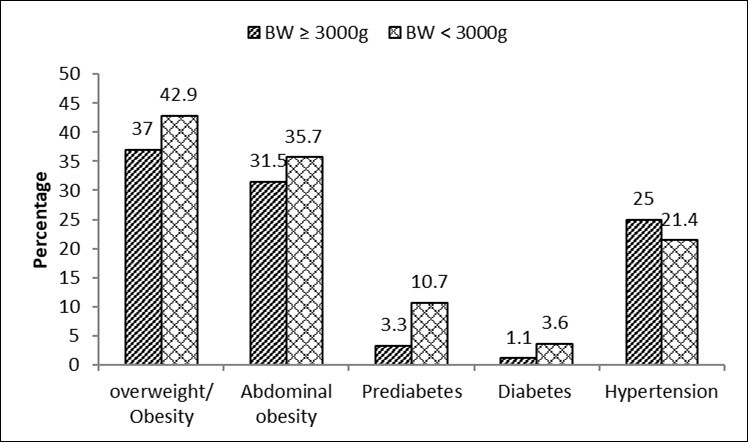
Hemodynamic and Metabolic Characteristics According to BW in Women (Table 2) and in Overweight/Obese Subjects (Table 3)
Mean aPWV was significantly (p=0.038) higher (on average +3 cm/s) in women with LBW compared to women with NBW (Table 2). Moreover, as compared to controls, women with LBW had significantly higher mean blood glucose levels, above the 126 mg/dL threshold defining diabetes mellitus; however, the observed difference was not statistically significant. In men, all hemodynamic and metabolic parameters were comparable between subjects with LBW and those with NBW (data not shown).
Table 2. Clinical, hemodynamic and biological data in women according to birth weight| ≥ 3000 gN=43 | < 3000 gN=12 | P | |
| Age (years) | 24 ±5 | 29 ±6 | 0.036 |
| BMI (kg /m2) | 25.7 ±5.6 | 26.1 ±4.7 | 0.768 |
| Glycemia (mg/dl) | 103 ±10.6 | 148 ±20.0 | 0.213 |
| aPWV (m/s) | 5.3 ±0.3 | 5.6 ±0.4 | 0.038 |
| SBP (mmHg) | 125 ±12 | 120 ±8 | 0.262 |
| DBP (mmHg) | 76 ±12 | 76 ±6 | 0.386 |
| MAP (mmHg) | 99 ±10 | 94 ±7 | 0.262 |
| PP (mmHg) | 38 ±9 | 36 ±8 | 0.698 |
| HR (bpm) | 77 ±11 | 78 ±10 | 0.807 |
As shown in Table 3, mean blood glucose levels were significantly higher in overweight/obese individuals with LBW, also above the threshold defining diabetes mellitus, compared to overweight/obese subjects with NBW (P=0.003). Furthermore, the aPWV was slightly higher (on average +2 cm/s) in overweight/obese subjects with LBW, albeit non-significantly (p=0.15).
Table 3. Anthropometric, hemodynamic and biologic parameters according to birth weight in overweight/obese participants (Body mass index ≥ 25 kg/m²)| BW ≥ 3000 g N=34 | BW < 3000 g N=12 | P | |
| Age ( years ) | 27 ±5 | 31 ±7 | 0.146 |
| BMI (kg /m 2 ) | 29.9 ±4.0 | 29.0 ±2.8 | 0.634 |
| Glycemia (mg /dl) | 87 ±1.1 | 154 ±19.7 | 0.003 |
| aPMW (m/s) | 5.5 ±0.3 | 5.7 ±0.4 | 0.146 |
| SBP ( mmHg ) | 134 ±11 | 127 ±10 | 0.127 |
| DBP ( mmHg ) | 80 ±13 | 81 ±9 | 0.557 |
| MAP ( mmHg ) | 104 ±10 | 102 ±9 | 0.507 |
| PP ( mmHg ) | 41 ±9 | 39 ±10 | 0.438 |
| HR ( mmHg ) | 76 ±12 | 73 ±10 | 0.556 |
Independent Determinants of Pulse Wave Velocity in the Overall Study Population and in Age Groups
In multivariate regression analysis performed in all participants (Table 4), age, BMI, male sex and MAP were independently associated with premature aortic aging assessed by PWV (Model 1). Birth weight adjusted for gestational age did not show a significant positive association with PWV when adjusting for age and sex (Model 2), and for age, sex, BMI and MAP (Model 3). For multiple regression in both two age groups (Table 5), PWV was independently associated with age and MAP, while in youngest age group (18-27 years), BMI and male sex were also independently related to early aortic aging (All p<0.05). Birth weight was not significantly and independently associated with PWV in the both two age groups when adjusting for covariates in Model 1, Model 2 and Model 3, respectively. Of note, in youngest age group, heart rate emerged as independent determinant of PWV after adjusting for age and sex (Modele2), and for age, sex, BMI and MAP (Table 3).
Table 4. Multiple regression with pulse wave velocity as dependent variable in all participants| Model 1 | Model 2 | Model 3 | ||||
| Variables | β (IC à 95%) | p | β (IC à 95%) | p | β (IC à 95%) | p |
|---|---|---|---|---|---|---|
| Age (years) | 0.07 (0.05; 0.08) | < 0.001 | 0.06 (0.05; 0.08) | < 0.001 | 0.03 (0.02; 0.05) | < 0.001 |
| Male sex | 0.24 (0.04; 0.44) | 0.022 | 0.21 (0.05; 0.38) | 0.011 | 0.15 (0.03; 0.26) | 0.011 |
| BW (kg) | 0.07 (0.45; -0.11) | 0.761 | 0.12 (-0.03; 0.26) | 0.117 | 0.05 (-0.04; 0.15) | 0.265 |
| MAP (mmHg) | 0.04 (0.03; 0.05) | < 0.001 | 0.03 (0.03; 0.04) | < 0.001 | 0.03 (0.02; 0.04) | < 0.001 |
| BMI (kg/m²) | 0.46 (0.27; 0.66) | < 0.001 | 0.04 (0.02; 0.06) | < 0.001 | 0.02 (0.01; 0.04) | 0.002 |
| HR (bpm) | 0,003 (-0,01; 0,01) | 0,504 | 0,01 (0,00; 0,02) | 0,047 | 0,001 (-0,01; 0,01) | 0,835 |
| Glycemia (g/L) | -0.06 (-0.15; 0.03) | 0.205 | -0.06 (-0.15; 0.03) | 0.205 | -0.03 (-0.10; 0.03) | 0.273 |
| Age 18 - 27 | Age 28 - 42 | |||||||||||||||
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |||||||||||
| Variables | β | p | β | p | β | p | β | p | p | β | β | p | p | β | β | p |
| Age (years) | 0,05 | 6 | 0,05 | 12 | 0,01 | 418 | 0,09 | 1 | 1 | 0,10 | 0,10 | < 0,001 | < 0,001 | 0,05 | 0,05 | 1 |
| Male sex | 0,25 | 17 | 0,21 | 36 | 0,16 | 24 | -0,10 | 579 | 579 | 0,34 | 0,34 | 37 | 37 | 0,23 | 0,23 | 35 |
| BW (kg) | 0,00 | 968 | 0,06 | 542 | 0,02 | 730 | 0,18 | 0,18 | 257 | 257 | 0,19 | 0,19 | 120 | 120 | 0,06 | 461 |
| MAP (mmHg) | 0,03 | < 0,001 | 0,03 | < 0,001 | 0,03 | < 0,001 | 0,05 | 0,05 | < 0,001 | < 0,001 | 0,04 | 0,04 | < 0,001 | < 0,001 | 0,04 | < 0,0001 |
| HR (bpm) | 5 | 346 | 12 | 16 | 12 | 16 | 5 | 5 | 606 | 606 | -0,03 | -0,03 | 726 | 726 | -3 | 534 |
| BMI (kg/m²) | 0,05 | < 0,001 | 0,06 | < 0,001 | 0,03 | 3 | 0,03 | 0,03 | 51 | 51 | 0,03 | 0,03 | 58 | 58 | 0,02 | 54 |
| Glycemia (g/L) | -0,05 | 287 | -0,06 | 223 | -0,03 | 285 | 0,59 | 0,59 | 468 | 468 | -0,29 | -0,29 | 675 | 675 | -0,79 | 63 |
Discussion
The findings of this observational study suggest that LBW can have deleterious late effects on arterial distensibility, but mainly in women who might suffer premature vascular aging. However, age, sex, BMI and MAP emerged as independent determinants of PWV, unlike BW adjusted for gestational age, which was not independently associated with EVA. Our results also show that individuals with LBW have an increased risk of glucose homeostasis abnormalities and obesity in late adolescence and adulthood. To our knowledge, this study is the first to evaluate the long-term effects of LBW on cardiometabolic risk in Cameroonian subjects.
Evidence suggests that unfavorable in utero environment, induced by a (quantitative and/or qualitative) fetal undernutrition or maternal placental insufficiency, may program the fetus, via epigenetic effects in particular, to subsequent development of CV and metabolic diseases, and to risk factors such as abnormal PWV, obesity and T2DM as phenotypic hallmarks 1, 21, 22, 23.
To provide further insight into possible pathophysiological mechanisms linking LBW to CVD risk in adulthood, we measured aortic PWV which has previously been shown to be a powerful marker of EVA 1, 2, 3 and an independent determinant of CV morbidity and mortality 16, 17. Age and BP are two major determinants of arterial stiffening 1, 2, 3. This was also the case in this study, in which we found that age and SBP were independently associated with PWV, reflecting aortic elasticity loss as a result of chronological aging and hypertension. Stiffening of large arteries, a major consequence of aging, results from alteration of their viscoelastic properties following wall remodeling (from eg. accumulation of calcium, decrease in elastin content, and increase in collagen). This leads to increased arterial stiffness (PWV), with rapid and early wave reflections from peripheral arteries towards the aorta, leading to increased SBP, PP and decreased DBP 15, 16. Consistent with previous observations 1, 17, 18, BMI and male sex emerged as independent determinant of PWV, even in the whole study populations, and especially in the youngest participants age group, 8-27 years old. Moreover, in youngest age group, HR shows significant positive association with PWV after adjusting for age, sex, BMI and MAP. This finding was in keeping with previous observations in young adults 1, 19.
Beyond age, several CV risk factors such as LBW may also promote premature aging of the aorta 1, 2, 3. Although our study did not find an independent association between LBW and PWV, likely as a result of the small sample size of the study, we nevertheless found that in young Cameroonian adults with a history of LBW, overweight and obesity were significantly associated with an increased PWV, even after adjusting for age and MAP. The association between LBW and PWV remains debated. Indeed, Miles et al 5 studied 882 participants in the United Kingdom, in whom increased arterial stiffness was not associated with LBW. By contrast, some studies have also documented stiffer arteries in apparently healthy children and adults with LBW 1, 8, 14, 30.
A major finding of the present study was the markedly increased PWV observed in women with LBW, that persisted even after adjustment for age and MAP compared to those with NBW. This observation corroborates that of Martyn et al 2 on 338 individuals born between 1939 and 1940, in whom intrauterine growth retardation was associated with increased aorto-iliac and femoro-popliteal-tibial PWV by the age of 50 years, regardless of adult lifestyles. The putative mechanisms underlying the increase in arterial stiffness in adulthood include be changes in angiogenesis during critical phases of intrauterine life caused by episodes of fetal growth inhibition and local hemodynamic anomalies 1, 2, 3, 4 and early endothelial dysfunction as well as premature remodeling of large arteries, ascribed to mechanical and growth factors, fetal protein deficiency, and detrimental effects of cytokines and proteases 1, 2, 13, 14.
We also observed that the MAP of participants with LBW for gestational age was approximately 2 mmHg higher than that of NBW participants (≥ 3000g). However, this difference was not statistically significant. Nevertheless, this finding is consistent with data in the literature, which reported an association between LBW and increased BP in adulthood 1, 2, 3. However, this association is debated 8, 33, 34. In the present study the prevalence of hypertension was higher among participants with NBW compared to those with LBW. This finding is inconsistent with observational studies that reported an increased risk of adult-onset hypertension in Caucasians with LBW 1, 2, 3, 4, and also with data from a study conducted in Nigeria, showing a high prevalence of hypertension in participants born during the Biafra war (1968-1970) and exposed to severe famine and materno-fetal and infantile malnutrition, compared with controls born in the same region just before the war, i.e. between 1965-1967 31. Our findings, although based on a small sample of subjects, are nevertheless in line with data from international epidemiological surveys 8, 9, 10.
A component of hypertension in adulthood was associated with unfavorable intrauterine conditions in mid- and late gestation, leading to disproportionate fetal growth 1, 2, 3, 4. The presumed role of fetal and/or early childhood malnutrition in driving the late burden of hypertension has not been examined in Africa, neither in Cameroon nor in other sub-Saharan African countries. Further large-scale studies are needed to better assess the impact of uterine malnutrition and LBW on the burden of hypertension in adulthood.
Of note, participants with LBW had an increased prevalence of overweight/obesity and visceral obesity. Our findings are consistent with previous observations showing a greater propensity to develop central and visceral obesity in adulthood in subjects with LBW 20, 21, 23, 31, 35, 36. Despite LBW and substantial risk of early postnatal underweight, former fetal-malnourished fetuses have higher subsequent obesity indices than controls with NBW, suggesting potential catch-up growth in adolescence of early adulthood. The latter, apparently beneficial, probably reflects rapid weight gain associated with poor linear growth, defining a phenotype of "obese stunted" subject with unfavorable body composition, including fat accumulation (especially visceral) and sarcopenia without height gain 23, 26, 31, 35, 36.
Another relevant finding of the present study is the high rate of glucometabolic abnormalities in subjects with LBW, globally and particularly in those with overweight/obesity in adulthood. This finding is in line with previous observations showing that subjects with LBW are at increased risk for incident metabolic syndrome 21, 22, 23 and T2DM 22, 37, 38, 39. This suggests that insulin resistance acquired in the context of visceral obesity is insufficiently compensated by compensatory hyperinsulinemia in many of these subjects with LBW, and that their insulin-secretory function is also less efficient in counteracting these effects of obesity 23, 37, 39. Experimental models suggest that protein deficiency resulting from materno-fetal nutritional imbalance may lead to developmental programming of carbohydrate homeostasis abnormalities in adulthood 38, 40.
Study Limitations
This study has several obvious limitations. First, this was a cross-sectional study with limited sample size and hence limited statistical power to detect magnitude of the effects of BW on risk of CV and metabolic diseases and exhibiting their associated major risk factors in adulthood in the general Cameroonian population. Further studies are needed, relying on larger samples size and including other regions of Cameroon. Second, several potentially confounding factors, such as socio-economic status, lipid profile, glycated hemoglobin, insulinemia, c-reactive protein, were not assessed due to financial and logistical constraints. Last, BW represents a single measurement and does not account for in utero growth trajectory and is not adjusted to parents’ heights. Proportionality indices assessing body size including body length, BMI at birth, and/or birth’s ponderal index (birth weight/birth length³), more accurately reflect fetal growth trajectory and may help differentiate fetuses who are constitutionally small for gestational age from fetuses who failed to reach their in utero growth potential, were not available for this survey 40. A strength of the present study is its originality and its design as stringent as possible, and the use of validated standard procedures to assess the variables of interest.
Conclusion
This study suggests that, whereas LBW is associated with increased aortic stiffness in young adulthood, mainly in women, the association was predominantly driven by age and blood pressure. Once adult, subjects with LBW exhibited higher obesity indices and poorer glucose homeostasis. Further large-scale studies are needed to assess the impact of BW and body size on arterial stiffness and metabolic disorders in later life.
References
- 1.Sperling J, Nilsson P M. (2020) Does early life programming influence arterial stiffness and central hemodynamics in adulthood?. , J Hypertens 38(3), 481-488.
- 2.Martyn C N, Greenwald S E. (1997) Impaired synthesis of elastin in walls of aorta and large conduit arteries during early development as an initiating event in pathogenesis of systemic hypertension. , Lancet 350(9082), 953-955.
- 3.Stock K, Schmid A, Griesmaier E, Gande N, Hochmayr C et al. (2018) The impact of being born preterm or small for gestational age on early vascular aging in adolescents. , J Pediatr 201, 49-54.
- 4.Barker D J, Gluckman P D, Godfrey K M, Harding J E, Owens J A et al. (1993) Fetal nutrition and cardiovascular disease in adult life. Lancet Lond Engl. 341(8850), 938-41.
- 5.Miles K L, McDonnell B J, Maki-Petaja K M, Yasmin N, Cockcroft J R et al. (2011) The impact of birth weight on blood pressure and arterial stiffness in later life: the Enigma Study. J Hypertens. 29(12), 2324-31.
- 6.Rondó P H, Lemos J O, Pereira J A, Oliveira J M, Innocente L R. (2008) Relationship between birthweight and arterial elasticity in childhood. , Clin Sci (Lond) 115(10), 317-26.
- 7.Law C M, Shiell A W. (1996) Is blood pressure inversely related to birth weight? The strength of evidence from a systematic review of the literature. , J Hypertens 14(8), 935-41.
- 8.Gamborg M, Byberg L, Rasmussen F, Andersen P K, Baker J L. (2007) NordNet Study Group. Birth weight and systolic blood pressure in adolescence and adulthood: meta-regression analysis of sex- and age-specific results from 20 Nordic studies. , Am J Epidemiol 166(6), 634-45.
- 9.Lawlor D A, Leon D A, Rasmussen F. (2007) Growth trajectory matters: interpreting the associations among birth weight, concurrent body size, and systolic blood pressure in a cohort study of 378,707 Swedish men. , Am J Epidemiol 165(12), 1405-12.
- 10.Huxley R R, Shiell A W, Law C M. (2000) The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. , J Hypertens 18(7), 815-31.
- 11.Barker D J, Winter P D, Osmond C, Margetts B, Simmonds S J. (1989) Weight in infancy and death from ischaemic heart disease. Lancet Lond Engl. 2(8663), 577-80.
- 12.Visentin S, Grumolato F, Nardelli G B, B Di Camillo, Grisan E et al. (2014) Early origins of adult disease: low birth weight and vascular remodeling. , Atherosclerosis 237(2), 391-9.
- 13.Rossi P, Tauzin L, Marchand E, Boussuges A, Gaudart J et al. (2011) Respective roles of preterm birth and fetal growth restriction in blood pressure and arterial stiffness in adolescence. , J Adolesc Health Off Publ Soc Adolesc Med 48(5), 520-2.
- 14.Barker D J, Osmond C, Simmonds S J, Wield G A. (1993) The relation of small head circumference and thinness at birth to death from cardiovascular disease in adult life. , BMJ 306(6875), 422-6.
- 15.O’Rourke M F, Staessen J A, Vlachopoulos C, Duprez D, Plante G E. (2002) Clinical applications of arterial stiffness; definitions and reference values. , Am J Hypertens 15(5), 426-44.
- 16.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank J K et al. (2012) Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. , J Hypertens 30(3), 445-8.
- 17.AlGhatrif M, Strait J B, Morrell C H, Canepa M, Wright J et al. (2013) Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertens Dallas Tex. 62(5), 934-41.
- 18.O’Rourke M. (1990) Arterial stiffness, systolic blood pressure, and logical treatment of arterial hypertension. Hypertens Dallas Tex. 15(4), 339-47.
- 19.McEniery C M, Hall IR Yasmin, Qasem A, Wilkinson I B, Cockcroft J R. (2005) Normal Vascular Aging: Differential Effects on Wave Reflection and Aortic Pulse Wave Velocity: The Anglo-Cardiff Collaborative Trial (ACCT). , J Am Coll Cardiol 46(9), 1753-60.
- 20.Barker D J, Hales C N, Fall C H, Osmond C, Phipps K et al. (1993) Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. , Diabetologia 36(1), 62-7.
- 21.Li Y, Jaddoe V W, Qi L, He Y, Wang D et al. (2011) Exposure to the Chinese famine in early life and the risk of metabolic syndrome in adulthood.DiabetesCare. 34(4), 1014-1018.
- 22.Forsén T, Eriksson J, Tuomilehto J, Reunanen A, Osmond C et al. (2000) The fetal and childhood growth of persons who develop type 2 diabetes. , Ann Intern Med 133(3), 176-82.
- 23.Ibáñez L, Ong K, F De Zegher, Victoria Marcos M, Del Rio L et al. (2003) Fat distribution in non-obese girls with and without precocious pubarche: Central adiposity related to insulinaemia and androgenaemia from prepuberty to postmenarche. , Clin Endocrinol (Oxf) 58(3), 372-9.
- 24.Njom Nlend AE, Zeudja C, Ndiang S, Nga Motaze A, Ngassam L et al. (1998) Trends in neonatal mortality of very-low-birth-weight infants between. and 2013 in Essos Hospital , Yaoundé, Cameroon] 23(9), 895-8.
- 25.Lemogoum D, L Van Bortel, Najem B, Dzudie A, Teutcha C et al. (2004) Arterial stiffness and wave reflections in patients with sickle cell disease. Hypertension. 44, 924-9.
- 26.Franssen P M, Imholz B P. (2010) Evaluation of the Mobil-O-Graph new generation ABPM device using the ESH criteria. Blood Press Monit. 15, 229-231.
- 27.Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A et al. (2013) ESH/ESC Guidelines for the management of arterial hypertension. The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 31, 1281-1357.
- 28.Clayton P E, Cianfarani S, Czernichow P, Johannsson G, Rapaport R et al. (2007) Management of the child born small for gestational age through to adulthood: a consensus statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. , J Clin Endocrinol Metab 92(3), 804-10.
- 29. (2010) American Diabetes Association (ADA). Standards of medical care in diabetes-2010. , Diabetes Care 33, 11-61.
- 30.Montgomery A A, Ben-Shlomo Y, McCarthy A, Davies D, Elwood P et al. (2000) Birth size and arterial compliance in young adults. , The Lancet 355(9221), 2136-7.
- 31.Hult M, Tornhammar P, Ueda P, Chima C, Bonamy A K et al. (2010) Hypertension, diabetes and overweight: looming legacies of the Biafran famine. PLoS One. , Oct 5(10), 13582.
- 32.Law C M, Shiell A W. (1996) Is blood pressure inversely related to birth weight? The strength of evidence from a systematic review of the literature. , J Hypertens 14(8), 935-41.
- 33.A-KE Bonamy, Norman M, Kaijser M. (2008) Being Born Too Small, Too Early, or Both: Does it Matter for Risk of Hypertension in the Elderly?. , Am J Hypertens 21(10), 1107-10.
- 34.DJ1 Barker, Bull A R, Osmond C. (1990) Simmonds SJ Fetal and placental size and risk of hypertension in adult life. , BMJ 301(6746), 259-62.
- 35.Lurbe E, Carvajal E, Torro I, Aguilar F, Alvarez J et al. (2009) . Influence of Concurrent Obesity and Low Birth Weight on Blood Pressure Phenotype in Youth. Hypertension 53, 912-917.
- 36.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H et al. (2005) Being big or growing fast: systematic review of size and growth in infancy and later obesity. , BMJ 331, 929.
- 37.JG1 Eriksson, Forsén T, Tuomilehto J, Osmond C, Barker D J. (2003) Early adiposity rebound in childhood and risk of Type 2 diabetes in adult life. , Diabetologia 46(2), 190-4.
- 38.Barker D J, Hales C N, Fall C H, Osmond C, Phipps K et al. (1993) Type 2 (noninsulin dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reducal fetal growth. , Diabetologia 36, 62-67.
