Abstract
This short review tracks advances in differential pulse voltammetry for in vivo applications. It summarizes electrode designs, analyte scope, and calibration strategies, and outlines challenges for selectivity and biocompatibility.
Author Contributions
Academic Editor: Zhe-Sheng Chenz, Professor, Department of Pharmaceutical Sciences, College of Pharmacy and Allied Health Professions, St. John’s University, United States.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2020 Francesco Crespi
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Background
In 1924 Heyrovsky found that current at a mercury electrode was not directly proportional to the applied voltage, but there was presence of an extra-current determined by the oxidisable chemicals present in the solution. Such extra current, that is proportional to the concentration of the compound(s) oxidized and/or reduced, is called polarographic current when obtained at a mercury electrode, is called voltammetric current when obtained at all other types of electrodes 1, 2.
Different types of voltammetric techniques are available the most common of which are chrono-amperometry linear voltammetry, cyclic voltammetry, and pulse voltammetry 3, 5.
These methodologies are mainly based on the application of a “dynamic” oxidation or oxido-reduction (ox – red) potential and the resulting analysis of electrons “freed” by the chemical(s) under analysis (see Figure 1).
Figure 1.voltammetry principle and schematic representation of the carbon fiber micro electrode (modified from ref 5 with permission).
Technique
Voltammetric measurements are taken with a three-electrode potentiostat system made of a silver/silver chloride (Ag/AgCl) reference electrode, a copper or silver wire auxiliary (counter) electrode both approximately 100 μm in diameter and a working electrode (see Figure 2). Nowadays, the working electrode is mainly a carbon fiber micro electrode (Figure 1).
Figure 2.schematic representation of the three-electrode potential system left and the reference and auxiliary electrodes (modified from ref 6 with permission).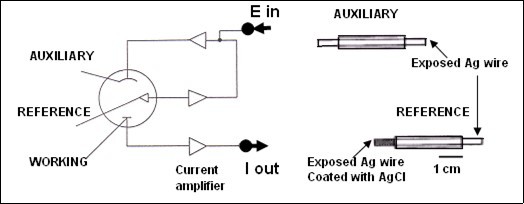
Electrodes for Voltammetry
Different types of voltammetric electrodes have been developed since 1969, the most performing type appear to be the carbon based electrodes and in particular the carbon fiber - micro electrode (µCFE) (see Figure 3) 3, 5, 6.
Figure 3.schematic representation of the evolution upon time of the manufacture of the carbon fiber micro electrode used as working electrode for voltammetry. A: is the conductive wire, B: resin alone or D: mixed with graphite paste. E is the carbon fiber that can vary between 6, 7, 10, 30 μm diameter. * is the protruding tip of the carbon fiber from the glass capillary (C). The length of the tip can vary from 0.1mm up to 1- 2mm in function of the size of the brain area monitored or the biological tissue analyzed, i.e. aortic(11), gastric tissue (12) (modified from ref 5 with permission).
The association of voltammetry with these electrodes become an electrochemical methodology allowing continuous, in real time and in situ detection of oxidizable chemicals.
The turning point of the use of these micro-sensors has been the application of a variety of electrical pre-treatments that are applied to the sensors before use. This has indeed improved drastically sensitivity and selectivity for analysis of electro-active chemicals and this in particular when the electrochemical methods of normal pulse as well as differential pulse voltammetry are employed 7, 8, 9, 10. Then, evolutions on pre-treatment of the µCFE have also been proposed. In particular, in addition to the electrical pre-treatment, a chemical pre-treatment consists in coating the protruding active tip of the micro sensor with Nafion (Sigma). Nafion is a sulphonated polymer repelling acids while attracting bases as it is negatively charged. This electrode is then called Nafion µCFE and it allows selective detection of dopamine and serotonin in vitro as well as in vivo with a greater sensitivity for the latter 13. Further development of such chemical pre-treatment is the coating with a mixture of Nafion and Crown ether (Sigma). The reresulting sensor is called NaCro µCFE and shows an improved sensitivity for the selective detection of these amines 14 and in particular that of dopamine 15.
Differential Pulse Voltammetry (DPV)
DPV combines aspects of chronoamperometry and linear sweep voltammetry and exhibits high selectivity and sensitivity. Small voltage pulses of a constant amplitude (20-50 mV) are superimposed 3-5 times per second upon a linear voltage ramp (see Figure 4). The current is sampled immediately before (iA) a pulse and subtracted from the current at the end of the pulse (iB), then the difference iB - iA is expressed in terms of potential. This consents to DPV to combines the main advantage of chronoamperometry (suppression of charging current) with the resolution of sweep voltammetry, as it performs a local differentiation of the voltammogram obtained by linear voltammetry. The overlap between two oxidisable compounds is eliminated providing that they oxidize at sufficiently distinct potentials (at least 50-100 mV between both). Thus, the oxidation of a compound produces a sharp peak rather than the broad peak or plateau of linear sweep voltammetry, resulting in higher resolution 16.
Figure 4.In Differential pulse voltammetry the applied potential is A: a linearly increasing ramp upon which small pulses of amplitude ΔV are superimposed. B: two measurements are made for each pulse; one just before the pulse iA and one just before the end of the single pulse iB, to yield the differential current value ΔI. C: the differential current ΔI is reported against the applied potential V to give the peak-shaped voltammogram (peak). D: in vivo, i.e. in rat striatum, DPV monitoring the peaks of dopamine DA and serotonin 5 at approximately 10mV and 200mV, respectively h: size of the peak in nanoAmperes [nA. 
The association of DPV with pre-treated µCFE appears to be the best methodology for rapid in situ analysis of electro-active compounds. No other combination of electrode and voltammetric method allows the same sensitivity with high resolution between oxidizable chemicals and in particular:
i)in vitro, with the active tip of the sensor immersed in buffered solution 7, 17, 3;
ii) ex vivo, with the active tip of the sensing electrode in contact with several tissue such as brain slices 18, 19, the endothelium of rat aortic rings for detection of nitric oxide and nitrites 11, 20, 21 or in blood, and in particular in platelet-rich plasma (PRP) and/or in isolated platelet (IP) 22 as well as in gastric tissue for detection of peptides 12;
iii)in vivo, in brain extracellular fluid when the sensor is stereotactically implanted in discrete brain areas of anesthetized as well as freely moving animals 4, 10, 23. In particular, in vivothe DPV methodology associated with carbon fiber micro electrodes (DPV-μCFE) becomes an advanced approach to monitoring changes in monoamine release and their metabolism. Indeed, the method fulfills many of the criteria required to monitor specific compounds in the extracellular fluid 5 in brief:
The undersized probe allows sampling a region of approximately 10-6 mm3 volume i.e. there is high anatomical resolution of the site of measurement within discrete brain areas of rodents, with minimal damage to the nervous tissue.
The method allows rapid, repeated measurements with accurate time resolution in vivo, in situ in real time without requiring perfusion, sample preparation, chromatographic separation or radio-labeled transmitter supplies. This is the fundamental difference between voltammetry and the perfusion techniques such as micro-dialysis 24, 25.
The association DPV - μCFE can be performed in vivo in conscious freely moving animals. This solves the problems associated with anesthetics and allows correlations between neuronal activity and behavior 5, 6.
Pharmacological experiments performed with DPV - μCFE have indeed demonstrated that the following chemicals can be selectively monitored in vivo in brain areas:
Ascorbate, noradrenaline and/or dopamine and the metabolites DOPAC, homovanillic acid, 3-methoxytyramine 26, 27, 28, 29;
Uric acid, 30;
5-OH-indoles (i.e.serotonin and its metabolite 5-OH-indolacetic acid) 8, 10, 13, 23.
In addition to the detection of monoamine release and their metabolism, in particular those of dopamine and serotonin, other electro-active chemicals have been successively detected with the association DPV - μCFE in vitro as well as in vivo as shown in figure 5. In particular, melatonin 31, 32 nitric oxide and nitrites 21, 33, 34 have been monitored between 500 and 700mV oxidation potential. Furthermore, neuropeptides containing electro-active amino acids such as tryptophan, cysteine, tyrosine appear to be electrochemically active in vitro; their oxidation potentials occur between +600 and +900mV 35, 36, 37 so that they are well demarcated from the selective DPVoltammetric oxidation peaks linked to the monoamines, the related metabolites and the other compounds mentioned above. Thus, the associated peptidergic oxidation signal has been called Peak 5 and it has been demonstrated that it is linked to the in situ oxidation of somatostatin (SRIF) 35, 37, cholecystokinin (CCK) 38, 39, 40 or neuropeptide Y (NPY) 41 depending on the discrete brain region analyzed. Hydrogen peroxide (H2O2) was also successively monitored in vivo, in situ and in real time in rat brain at approximately 1000mV 42.
Figure 5.Electro-active compounds measurable at selective oxidation potentials in vitro as well as in vivo with the association DPV - µCFE (modified from ref 5 with permission).
Variations of the pulse polarography technique have also been proposed. In particularDifferential Square Pulse Conditioning Voltammetry has been introduced since it is permitting longer “life” to the micro sensor when used in vivo 43, 44. Another variant is Short Range Differential Pulse Polarography that couples sensitivity and selectivity with very rapid measurement of endogenous chemicals 45, 46. Again, Differential Pulse Stripping Voltammetry, characterized by the addition to a DPV scan of a conditioning potential followed by a cleaning potential, permits nearly continuous measurements without loss of sensitivity. This is a clear advantage when one need to combine the analysis of behavior with related changes of neurotransmitters, for instance.
Finally, a very recent achievement of the association DPV - μCFE is the evidence of the feasibility of monitoring Lactic Acid both in vitro and in vivo in the frontal cortex of rodents at the selective oxidation potential +1.5 Volts 47 (see Figure 5).
It appears therefore evident that this electrochemical methodology is still evolving in detecting a variety of chemicals, at the same time as presenting a range of advantages over methods based on the preparation of samples and/or separation steps. Indeed, it allows rapid, direct, concomitant finding of different chemicals based upon specific oxidative (or red-ox) potentials either in vitro, ex vivo and in vivo conditions 48.
Such a flexibility of application is illustrated by the feasibility to couple this methodology with behavioral observations 49, with electrophysiological recordings, for example of the sleep-awake cycle 23 as well as with in vivo cell firing 36, 50, 51.
A particular example of such flexibility of utilization is the feasibility to apply the methodology in physiologic as well as pathological conditions, thus proposing selective mechanisms of actions of the neurotransmitters that can be monitored in vivo, in situ and in real time. This, taken together with the recent improvement in the methodology permitting DPVoltammetric analysis in telemetric – wireless conditions, thus allowing electrochemical studies in absolutely freely moving conditions 52 may help in the understanding of cerebral diseases and possibly in the development of pharmacological approaches to tackle them 53, 54, 55, 56, 57, 58, 59, 60.
References
- 1.Adams R N. (1969) Application of modern electroanalytical techniques to pharmaceutical chemistry. , J. Pharm. Sci 58, 1171-1178.
- 2.Adams R N. (1969) Rapid voltage sweep methods at stationary electrodes In Electrochemistry at Solid Electrodes.Marcel Dekker,N.Y.
- 3.Shah A J, Crespi F, Heidbreder C. (2002) Amino acid neurotransmitters: separation approaches and diagnostic value. , [Review] Journal of Chromatography B: Analytical Technologies in the Biomedical & Life Sciences.781(1-2): 151-163.
- 4.Stamford J, Crespi F, Marsden C A. (1992) In vivo voltammetric methods for monitoring monoamine release and metabolism. In Practical Approach Series Monitoring Neuronal Activity. Irl Press at OxfordUniversityPress:Oxford,U.K 113-145.
- 5.Crespi F. (2013) Serotonin, how to find it...Review Article Xjenza Online -. , Journal of Malta Chamber of Scientist.http://www.mcs.org.mt/.Doi:http://dx.medra.org/10.7423/XJENZA.2.0214-22
- 6.Crespi F. (2014) Invasive or Non-Invasive Techniques and Sensors for Real Time In Vivo Sensing in the Brain" chapter in the book, Laboratory and Clinical Research: "Microelectrodes: Techniques, Structures for Biosensing and Potential Applications" Nova Biomedical Science Publishers, ISBN: 978-1-62948-721-2(e-book)Inc (Kin Fong Ley ED.).
- 7.Ponchon J L, Cespuglio R, Gonon F, Jouvet M, Pujol J F. (1979) Normal pulse polarography with carbon fiber electrodes for in vitro and in vivo determination of catecholamines Anal. , Chem 51(9), 1483-1486.
- 8.Martin K F, Marsden C A, Crespi F. (1988) In vivo electrochemistry with carbon fibre electrodes: principles and application to neuropharmacology. , Trends Anal. Chem 7(9), 334-339.
- 9.Self T, Crespi F. (1992) Electron microscopic and voltammetric analysis of carbon fibre electrode pretreatments. , Journal of Materials in Medicine 3, 418-425.
- 10.Crespi F. (1990) In vivo voltammetry with micro-biosensors for analysis of neurotransmitter release and metabolism. , Journal of Neuroscience Methods 34, 53-65.
- 11.Crespi F, Vecchiato E, Lazzarini C, Gaviraghi G. (2001) Electrochemical evidence that lacidipine stimulates release of nitrogen monoxide (NO) in rat aorta. , Neuroscience Letters 298, 171-174.
- 12.Crespi F. (2017) Central [CNS] and Peripheral [Gastric Tissue] Selective Monitoring of Somatostatin. (SRIF) with Micro-Sensor and Voltammetry in Rats: Influence of Growth Factors (GH, EGF). Biosensors 7(4), 53-63.
- 13.Crespi F, Martin K F, Marsden C A. (1988) Nafion coated carbon fibre electrodes combined with differential pulse voltammetry measure 5HT release in vivo. , Neuroscience 27, 885-896.
- 14.Baumeyer T, Dittrich J, Crespi F. (1993) Nafion-crown ether modified carbon fibre electrodes: new microbiosensors for detection of neurotransmitters in vitro and in vivo. , Electroanalysis 5(7), 565-570.
- 15.Mobius M, Crespi F. (1992) In vivo selective monitoring of basal levels of cerebral dopamine using voltammetry with NAFION-CROWN ETHER carbon fibre microsensors.Journal of. , Neuroscience Methods 42, 149-161.
- 16.Flato J B. (1972) The renaissance in polarographic and voltammetric analysis. , Analyt. Chem 44, 78-87.
- 17.Goutier W, Lowry J P, McCreary A C. (2016) Frequency-Dependent Modulation of Dopamine Release by Nicotine and Dopamine D1 Receptor Ligands: An In Vitro Fast Cyclic Voltammetry Study in Rat Striatum. , Neurochem Res 41, 945-950.
- 18.Bull D, Palij M, Millar J. (1990) Application of fast cyclic voltammetry to measurement of electrically evoked dopamine overflow from brain slices in vitro. , J. Neurosci. Methods 32, 37-44.
- 19.Rice M E, Patel J C, Cragg S J. (2011) Dopamine release in the basal ganglia. , Neuroscience 198, 112-137.
- 20.Cristofori P, Crivellente F, Faustinelli I, Turton J, Zancanaro C et al. (2004) Involvement of the NITROGEN MONOXIDE (NO) system in the anti-atherosclerotic potential of LACIDIPINE in Apo-E deficient mouse: A morphological, functional and electrochemical study. , Toxicologic Pathology 32, 493-499.
- 21.Rossetti Z, Crespi F. (2004) Inhibition of Nitric Oxide release in vivo by ethanol Alcoholism:. , Clinical and Experimental Research 28, 1-6.
- 22.Bianchi M, Moser C, Lazzarini C, Vecchiato E, Crespi F. (2002) Forced swimming test and fluoxetine treatment: in vivo evidence that Periphal 5-HT in rat platelet-rich plasma mirrors cerebral extracellular 5-HT levels, whilst 5-HT in isolated platelets mirrors neuronal 5-HT changes. , Experimental Brain Research 143, 191-197.
- 23.Crespi F, Jouvet M. (1983) Differential pulse voltammetry: parallel peak 3 changes with vigilance states in raphe dorsalis and raphe magnus of chronic freely moving rats and evidence for 5HT contribution to this peak after monoamine oxidase inhibitors. , Brain Research 272, 263-268.
- 24.Ungerstedt U, Hallström A. (1987) In vivo microdialysis - a new approach to the analysis of neurotransmitters. in the brain Life Sciences 41(7), 861-864.
- 25.Ortega J E, Meana J J, Callado L F. (2016) In Vivo Brain Microdialysis of Monoamines. In: Luján R., Ciruela F. (eds) Receptor and Ion Channel Detection in the Brain. Neuromethods, vol 110. , New York, NY
- 26.Buda M, Gonon F, Cespuglio R, Jouvet M, Pujol J F. (1981) In vivo electrochemical detection of catechols in several dopaminergic brain regions of anaesthetized rats. , European Journal of Pharmacology 73(1), 61-68.
- 27.Crespi F, Keane P. (1987) Analysis of extracellular DOPAC, HVA, 5-HIAA and ascorbic acid in rat striatum in vivo by DPV, effect of PCP, haloperidol and their co-administration. , Research Communications 19, 639-649.
- 28.Crespi F, Martin K F, Heal D J, Marsden C A, Buckett W R et al. (1989) Measurement of 3 methoxytyramine by in vivo voltammetry: evidence for differences in cerebral dopamine function in Balb/c and CBA mice. , Brain Research 500, 241-246.
- 29.Sanghera M, Crespi F, Martin K, Heal D J, Buckett W R et al. (1990) Biochemical and in vivo voltammetric evidence for difference in striatal dopamine levels in inbred strains of mice. , Neuroscience 39(3), 649-656.
- 30.Crespi F, Sharp T, Maidment N, Marsden C A. (1983) Differential pulse voltammetry in vivo, evidence that the uric acid contributes to the indole oxidation peak. , Neuroscience Letters 43, 203-207.
- 31.Crespi F, Ratti E, Trist D G. (1994) Melatonin, a hormone monitorable in vivo by voltammetry? The Analyst119. 2193-2197.
- 32.Crespi F. (2012) Influence of melatonin or its antagonism on alcohol consumption in ethanol drinking rats: a behavioural and in vivo voltammetric study. , Brain Research 1452, 39-46.
- 33.Crespi F, Campagnola M, Neudeck A, McMillan K. (2001) Can voltammetry measures nitrogen monoxide (NO) and/or nitrites?. , Journal of Neuroscience Methods 109, 59-70.
- 34.Crespi F. (2013) In vivo voltammetric evidence that cerebral nitric oxide (NO) is influenced by drugs of abuse: Is NO implicated in their neurotoxicity? RSC Adv.3, 9803–9808.This journal is @ The Royal Society of Chemistry.
- 35.Crespi F. (1991) In vivo voltammetry detection of neuropeptides with micro carbon fibre biosensors: possibile selective detection of somatostatin. , Analytical Biochemistry 194, 1-8.
- 36.Crespi F. (2002) In vivo voltammetry and concomitant electrophysiology at a single biosensor to analyse ischaemia, depression and drug dependence. , Journal of Neuroscience Methods 119, 173-184.
- 37.Crespi F. (2017) Central [CNS] and Peripheral [Gastric Tissue] Selective Monitoring of Somatostatin. (SRIF) with Micro-Sensor and Voltammetry in Rats: Influence of Growth Factors (GH, EGF). Biosensors 7(4), 53-63.
- 38.Crespi F. (1998) The role of cholecystokinin (CCK), CCK-A or CCK-B receptor antagonists in the spontaneous preference for drugs of abuse (alcohol or cocaine) in naive rats. , Methods Find Exp Clin Pharmacol 20(8), 679-97.
- 39.Crespi F, Corsi M, Reggiani A, Ratti E, Gaviraghi G. (2000) Involvement of cholecystokinin within craving for cocaine: role of cholecystokinin receptor ligands Expert Opin Investig Drugs 9(10),2249-2258.
- 40.Crespi F, Congestri F, Formenti F. (2019) Evidence that ethanol selectively alters dopamine and serotonin metabolism as well as peptidergic levels in CA3 hippocampus of spontaneously alcohol preferring rats. , Clin Res Trials.doi;10.15761/CRT.1000247 5.
- 41.Crespi F. (2011) Influence of Neuropeptide Y and antidepressants upon cerebral monoamines involved in depression: An in vivo electrochemical study. , Brain Research 1407, 27-37.
- 42.Crespi F. (2014) Hydrogen peroxide monitored in vivo, in situ and in real time in rat brain, is it a marker of central cholinergic dynamics?. , Analytical Methods 6, 1174-1181.
- 43.Baumeyer T, Dittrich J, Crespi F. (1990) Differential square pulse conditioning voltammetry: a new method for electrochemical analysis with micro-biosensors. , British Journal of Pharmacology 99, 260.
- 44.Crespi F, Baumeyer T, Dittrich J.Simultaneous in vivo monitoring of dopamine and serotonin by differential pulse conditioning voltammetry with Na-cro microbiosensors. , Fresenius Journal of Analytical Chemistry 341(10), 644.
- 45.F Neudeck A Crespi. (1992) Short Range Differential Pulse Polarography for fast, selective analysis of cerebral electroactive compounds in vivo. , British Journal of Pharmacology 107, 427.
- 46.Crespi F, Neudeck A. (1993) Short Range Differential Pulse Voltammetry for fast, selective analysis of basal levels of cerebral compounds in vivo. , Journal of Neuroscience Methods 50, 225-235.
- 47.Crespi F. (2019) Feasibility analysis of lactic acid detection by in vivo voltammetry in the rat brain: Influence of oxygen and carbon dioxide. Current Topics in. , Analytical Chemistry 11, 51-58.
- 48.Sanghavi B J, Wolfbeis O S, Hirsch T, Swam N S. (2015) Nanomaterial-based electrochemical sensing of neurological drugs and neurotransmitters. , Mikrochim. Acta 182, 1-41.
- 49.Crespi F. (2010) Is a divergent central serotonergic activity responsible for either despair or learning behavior in intact Wistar or Sprague-Dawley CD rats, respectively? A concomitant behavioral and electrochemical analysis. , PSYCHOLOGY 1, 1-11.
- 50.Formenti F, Sonntag V, Congestri F, Crespi F. (2009) . Dopamine D3 Receptor Antagonist SB-277011A Influences Cell Firing in the Rat VTA, Parallel Role with the Cannabinoid System in Addiction and Neuropsychiatry Disorders? The Open Neuropsychopharmacology Journal 2, 86-92.
- 51.Crespi F. (2009) Anxiolytics antagonize Yohimbine-induced central noradrenergic activity: a concomitant in vivo voltammetry – electrophysiology model of anxiety. , Journal of Neuroscience Methods 180, 97-105.
- 52.Crespi F. (2010) Wireless in vivo voltammetric measurements of neurotransmitters in freely behaving rats. , Biosensors and Bioelectronics 25, 2425-2430.
- 53.Crespi F, Jouvet M. (1982) Sleep and indolamine alterations induced by thiamine deficiency. , Brain Research 248, 275-283.
- 54.Crespi F. (1986) Influence of stress and of stress-related peptides on the cerebral metabolism of dopamine and serotonin measured in the striatum of conscious freely moving rats. Medicina Riv E.M.I. 6, 379-382.
- 55.Crespi F. (1989) Influence of forced immobilisation and of stress-related peptides on striatal DOPAC and 5HIAA voltammetrically detected in freely moving rats. , Regulatory Peptides 26(1), 65.
- 56.Crespi F, Mobius M, Wright I K. (1992) Isolation rearing of rats alters behaviour and release of 5-hydroxytryptamine and dopamine in the frontal cortex. , Experimental Brain Research 88, 495-501.
- 57.Crespi F. (1996) Concomitant in vivo electrophysiological and voltammetric analysis indicate that ascorbic acid is a biochemical index of early ischaemia. , Neuroscience Letters 215, 189-192.
- 58.Crespi F, Pietra C. (1997) Middle cerebral artery occlusion alters neurotransmitter activities in ipsilateral and contralateral rat brain regions: an ex vivo voltammetric study. , Neuroscience Letters 230, 77-80.
Cited by (8)
- 1.Gandarilla Ariamna Maria Dip, Matos Robert Saraiva, Barcelay Yonny Romaguera, da Fonseca Filho Henrique Duarte, Brito Walter Ricardo, 2022, Molecularly imprinted polymer on indium tin oxide substrate for bovine serum albumin determination, Journal of Polymer Research, 29(5), 10.1007/s10965-022-03022-5
- 2.Birgusova Eliska, Navratil Jiri, Dostalova Eliska, Ashrafi Amir M., Bytesnikova Zuzana, et al, 2025, TP53 detection based on electrochemical genosensors with different types of gold nanoparticles, Microchemical Journal, 209(), 112856, 10.1016/j.microc.2025.112856
- 3.Abd El-Raheem Hany, Alawam Abdullah S., Rudayni Hassan A., Allam Ahmed A., Helim Rabiaa, et al, 2025, Emerging Electrochemical Approaches for the Early Detection of Programmed Cell Death, ACS Omega, 10(31), 34106, 10.1021/acsomega.5c05652
- 4.P Shivakumar, K S Manjunatha Kumara, Bose Shubhankar Kumar, D H Nagaraju, 2022, Advances in Zinc and Magnesium Battery Polymer Cathode Materials, ACS Applied Energy Materials, 5(9), 10331, 10.1021/acsaem.2c01555
- 5.Meskher Hicham, 2023, Critical study about recent advanced materials and their electrochemical sensing of organic pollutants, Journal of Composites and Compounds, 5(15), 102, 10.61186/jcc.5.2.5
- 6.Ma Yuan, Deng Yuping, Xie Chao, Zhang Bingjing, Shen Boyu, et al, 2023, A Review of Electrochemical Electrodes and Readout Interface Designs for Biosensors, IEEE Open Journal of the Solid-State Circuits Society, 3(), 76, 10.1109/OJSSCS.2022.3221924
- 7.Bassous Nicole Joy, Rodriguez Ashly Corona, Leal Celina Ivonne Lomeli, Jung Hyun Young, Lee Chang Kee, et al, 2024, Significance of Various Sensing Mechanisms for Detecting Local and Atmospheric Greenhouse Gases: A Review, Advanced Sensor Research, 3(2), 10.1002/adsr.202300094
- 8.El-Nakib Heba E., Abdel Aziz Shimaa E., Schaletzky Julia, Ahmed Nermin S., 2025, Analytical Methodologies for Anti-Infective Orphan Drugs: A Comprehensive Review of FDA Approvals (2013–2023) Part 2, Critical Reviews in Analytical Chemistry, (), 1, 10.1080/10408347.2025.2541764
