Computational Systemic Biology for Toxicity Studies: A Mini Review of Previously Published Articles
Abstract
The strategy for safe drug discovery and development has limited clinical success as compared to wasted time and resources annually. This is due to the fact that the results of multiphase preclinical trials are less likely to make an accurate early prediction on the safety of test compounds to progress into the clinic as a valuable therapeutic agent. A lot of time and resources has been wasted in the multistage processes of drug discovery and development that does not work at the end of the procedure every year. During pre-marketing stage, for instance, the number of unsuccessful clinical trials are greater than the successful one because of safety issues.
A toxicity study at different stages of preclinical and clinical trials is a routine procedure to investigate the undesirable side effects of test compounds being manifested on the natural processes of living things. It deals with the effect and mechanism of toxicity of test compounds that triggers different biological responses on different organ systems. The biological responses that would be manifested as a result of interaction between the receptors and active molecules of a test compound could be desirable pharmacological effect or undesirable side effect or both responses are manifested simultaneously depending on the selectivity or specificity of the molecule of a test compound for its receptor subtype which makes safe drug discovery and development very challenging.
The response efficiency of the body (the net outcome of the body’s biological reaction against the side effect) would determine the potency of a test compound to manifest undesirable pharmacologic effect. In other words, the amount of a drug required to cause a biological harm or injury depends on the magnitude of the body’s biological reaction in which the immune response plays a great pharmacological role by neutralizing and harmonizing xenobiotics with the biological molecules. The dose of a test compound at 100 mg/kg body weight, for instance, could be lethal to some of the study animals while it is still non-lethal to some other study animals depending on the response efficiency of the body. The immune system is well connected to each and every biological systems of the body which allows it to detect undesirable side effects being manifested through immunoglobulins signalling and activation mechanisms. This complex communication network helps to localize the diverse side effects of a test compound being manifested on different organ systems into the immune system which makes a toxicity study relatively simple to monitor. The cellular immune system becomes active following the molecule-receptor interaction and start producing antibodies which is also known as immunoglobulins to protect bodily harm and destruction. Under normal biological circumstances, the amount of immunoglobulins produced by the cellular immune system following exposure to a test compound is proportional to the number of harmful molecules interacted with its receptor subtype. Thus, with the reference to the changes in the immune response against the administered dose, it would be able to deal with the diverse undesirable side effects of a test compound being manifested on treated study animals using computational systemic biology.
Author Contributions
Academic Editor: ANUBHA BAJAJ, Consultant Histopathology, A.B. Diagnostics, New Delhi, India.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2022 Yilkal Tariku Belay
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
No author has any associations that may represent a potential conflict of interest.
Citation:
Background
The strategy for safe drug discovery and development has limited clinical success as compared to wasted time and resources annually 1, 2. This is due to the fact that the results of multiphase preclinical trials are less likely to make an accurate early prediction on the safety of test compounds to progress into the clinic as a valuable therapeutic agent. There is no specific maximum effective dose and minimum lethal dose for every test compound that could be targeted to be considered during the period of the trial. Different doses of a test compound have different length of time to manifest its pharmacological or toxicological effect on study subjects depending on the molecular nature in which the undesirable side effect may not be manifested within the period of multiphase preclinical or clinical trials. In addition to that, many test compounds are not specific or selective in their action which also makes toxicity studies very complex 2. A lot of time and resources has wasted in the multistage processes of drug discovery and development that does not work at the end of all the procedures every year. During pre-marketing stage, for instance, the number of unsuccessful clinical trials are greater than the successful one because of safety issues 1, 2. Even if a drug reaches the market, post-marketing surveillance could still characterise critical adverse effects of a drug which was not observed during pre-clinical and clinical trials 1, 2. Considerable number of therapeutic agents are removed even after being on market for a long period of time because of their public health issues i.e. thalidomide and warfarin.
A toxicity study at different stages of preclinical and clinical trials is a regulatory procedure to investigate the undesirable side effects and mechanism of toxicity of test compounds on the natural processes of living things. Depending on the objective of a toxicity trial, an In vivo or In vitro study design would be employed to evaluate the toxicity of active molecules of test compounds that perhaps trigger a biological signal on different biological systems. The biological responses that would manifest as a result of interaction between a drug receptor and active molecules of a test compound could be desirable pharmacologic effect or undesirable side effect or both responses manifested simultaneously depending on the selectivity or specificity of a molecule of a test compound for its receptor subtype 2 & 7. For example, propranolol, a nonselective β-adrenoceptor blocker, has both desirable and undesirable effects on different biological systems 2, 7. It has a desirable therapeutic effect on the cardiovascular system where it acts as a useful antihypertensive agent by reducing cardiac output and vascular resistance while it has undesirable side effect on the respiratory system where it prevents β2-receptor-induced bronchodilation which may precipitate bronchoconstriction in susceptible individuals 2, 7. On the other hand, verapamil, a calcium channel blocker, is relatively selective by acting as a useful antihypertensive agent without causing bronchoconstriction or prevent bronchodilation which is potentially harmful to asthmatic patient 2, 7. These shows that other test compounds are more likely to have many targets that could lead into the manifestation of undesirable side effects on some biological systems while it acts as a useful agent on other biological systems depending on the selectivity or specificity of the molecules of a compound for a receptor subtype which makes drug discovery and development very challenging.
The response efficiency of the body (the net outcome of the body’s biological reaction against the side effect) would determine the potency of a test compound to manifest undesirable pharmacologic effect. In other words, the amount of a drug required to cause a biological harm or injury depends on the intensity of the body’s biological reaction in which the immune response plays a great pharmacological role by neutralizing and harmonizing xenobiotics with the biological molecules 3. The differences between the amount of doses required to manifest a biological harm or injury reflects how efficient the biological response of the body is against its side effect. For example, the dose of a test compound at 100 mg/kg body weight could cause deaths to some of the study animals while it is still non-lethal to some other study animals depending on the response efficiency of the body. The response efficiency of the body is primarily dependent on the viability of the different biological systems 4. The function of each biological system is interrelated in which the proper function of one is dependent on the other. This means that the deterioration of one biological system as a result of biological harm being caused by a test compound would lead to the deterioration of other body systems. For example, a test compound which causes dysfunction of the metabolic system would definitely supress the magnitude of the immune response.
The immune system has well-orchestrated network of communication to each biological systems of the body which allows it to detect the undesirable biological signals being manifested through signalling and activation mechanisms of immunoglobulins. 5, 11, 12. It is the control centre of the body’s security through its complex communication network to all organ systems by which it detects the harmful molecules of a test compound or antigen and activate the cellular component of the immune system to participate in the immune response against it. This complex communication network helps to localize the diverse side effects of a test compound being manifested on different organ systems into the immune system which makes toxicity studies relatively simple to monitor.
Immunoglobulins are cell signalling proteins embedded in the cell membrane with the biological power to detect the harmful molecules of a test compound and activate the cellular immune system to produce antibodies which is also known as immunoglobulins to protect bodily harm and destruction 5. Under normal biological circumstances, the amount of immunoglobulins produced by the cellular immune system following exposure to a test compound is proportional to the number of harmful molecules interacted with its receptor subtype 3, 13. Thus, with the reference to the changes in the immune response against the administered dose, it would be quite possible to deal with the diverse side effects of a test compound being manifested on treated study animals using a computational systemic biology which is explained in the next subtitle.
The response efficiency of the body against the side effects of each doses administered into Balb c mice has been computed using an integrated biology based data to be able to make an approximate figure on the severity of a biological harm being caused by a dose of a test compound 3. First, acute toxicity trial of different test chemicals has been conducted on Balb c mice to process an integrated biological data to be able to compute the toxic severity and toxic reaction rate of an administered dose using the computational method explained in the next subtitle (Table 2 and table 3) 3. The study has revealed that the response efficiency of treated Balb c mice with the lower doses was high as compared with the higher doses of test chemicals which could be a strong evidence that life has predetermined response efficiency against the undesirable side effects of xenobiotics (Table 2 and table 3). The immunoglobulins immune response was quickly declined against the higher doses administered into study Balb c mice as compared to lower doses which was evidenced at four hour after dosing (Table 4) 3. For example, the immunoglobulins immune response against 10 mg/kg body weight of chlorpyrifos pesticide was high (30 mg/L) as compared to 50 mg/kg and 90 mg/kg body weight of the same test compound which was low (20 mg/L and -10 mg/L respectively) 3. The toxic severity of tested doses at 10 mg/kg, 50 mg/kg and 90 mg/kg body weight was -0.98, -0.40 and 0.37 percent per second respectively which was computed using the corrected formula mentioned below (Table 3) 3. The results of different experiments have shown that the toxic severity has increased alongside with the levels of doses administered to study Balb c mice (Figure 1). This implies to the fact that the response efficiency of the body diminishes while the amount of a dose administered to a study animal increases. The negative values of the toxic severity of doses mentioned above and in table 3 reflects the response efficiency of treated Balb c mice which was still powerful to resist the undesirable side effect of a higher dose of the same test chemical. On the other hand, the positive value of toxic severity shows the response efficiency of treated Balb c mice which was already exhausted or declined its resistance to the undesirable side effect of an administered dose.
Figure 1.The toxic severity of tested doses at 10 mg, 50 mg and 90 mg/kg body weight prepared from each test chemicals (dichlorvos, chlorpyrifos and cypermethrin).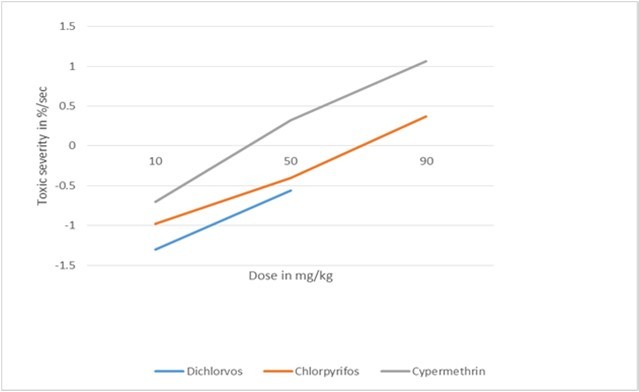
| Test chemicals | Tested doses | Body weight before dosing | Body weight 5 days after dosing |
| Dichlorvos | 10 mg/kg | 15.13 g | 13.37 g |
| 50 mg/kg | 17.63 g | 14.72 g | |
| 90 mg/kg | 16.42 g | X | |
| Chlorpyrifos | 10 mg/kg | 30.41g | 26.58 g |
| 50 mg/kg | 27.12 g | 23.37 g | |
| 90 mg/kg | 26.84 g | X | |
| Cypermethrin | 10 mg/kg | 28.42 g | 23.58 g |
| 50 mg/kg | 30.98 g | X | |
| 90 mg/kg | 28.24 g | X |
| t chemicals | Tested doses | Toxic reaction rate (r) in mg/sec |
| Dichlorvos | 10 mg/kg | -19.9 |
| 50 mg/kg | -9.9 | |
| 90 mg/kg | X | |
| Chlorpyrifos | 10 mg/kg | -29.9 |
| 50 mg/kg | -19.9 | |
| 90 mg/kg | 10.0 | |
| Cypermethrin | 10 mg/kg | -19.9 |
| 50 mg/kg | 10.0 | |
| 90 mg/kg | 30.0 |
Table 3. The toxic severity (s) of test chemicals computed at four hour after dosing.
| Test chemicals | Tested doses | Body weight | Toxic severity (s) in %/sec |
| Dichlorvos | 10 mg/kg | 15.13 g | -1.30 |
| 50 mg/kg | 17.63 g | -0.56 | |
| 90 mg/kg | 16.42 g | X | |
| Chlorpyrifos | 10 mg/kg | 30.41 g | -0.98 |
| 50 mg/kg | 27.12 g | -0.40 | |
| 90 mg/kg | 26.84 g | 0.37 | |
| Cypermethrin | 10 mg/kg | 28.42 g | -0.70 |
| 50 mg/kg | 30.98 g | 0.32 | |
| 90 mg/kg | 28.24 g | 1.06 |
Table 4. Serum immunoglobulins change (Δ Ig) four hours after treatment of Balb-c mice with different doses of test chemicals.
| Test chemicals | Tested doses in mg/kg | Quantitative immunoassay before treatment | Quantitative immunoassay four hours after treatment | Δ Ig serum conc. | ||
| IgG | IgM | IgG | IgM | Δ Ig | ||
| Dichlorvos | 10 | <1100 mg/L | 70 mg/L | <1100 mg/L | 90 mg/L | +20 mg/L |
| 50 | <1100 mg/L | 70 mg/L | <1100 mg/L | 80 mg/L | +10 mg/L | |
| 90 | X | X | X | X | X | |
| Chlorpyrifos | 10 | <1100 mg/L | 90 mg/L | <1100 mg/L | 120 mg/L | +30 mg/L |
| 50 | <1100 mg/L | 50 mg/L | <1100 mg/L | 70 mg/L | +20 mg/L | |
| 90 | <1100 mg/L | 90 mg/L | <1100 mg/L | 80 mg/L | -10 mg/L | |
| Cypermethrin | 10 | <1100 mg/L | 70 mg/L | <1100 mg/L | 90 mg/L | +20 mg/L |
| 50 | <1100 mg/L | 80 mg/L | <1100 mg/L | 70 mg/L | -10 mg/L | |
| 90 | <1100 mg/L | 80 mg/L | <1100 mg/L | 50 mg/L | -30 mg/L | |
X Represents treated mouse which died much earlier than the time for blood specimen collection.
As it has been evidenced earlier, the molecules of many test compounds are more likely to bind to more than one type of receptors, thereby manifesting diverse biological responses on study animals 2 & 7. Therefore, it requires an integrated biology based approach to try to consider the various adverse effects being manifested on different organ systems and evaluate the response efficiency of the body as a whole. The specificity and affinity of a receptor subtype for binding to the molecule of a drug would determine the type and magnitude of a biological response 8. Therefore, computational method for systemic biology is crucial to procure an inclusive and reliable data and compute the response efficiency of the body against the dose administered into a study animal to be able to clearly decide the pharmacological effect of a test compound.
Components of the Computational Method for a Toxicity Study
Acute toxicity study has been conducted on Balb c mice using different levels of doses of test extracts from the seed of traditional herb known as Aristolochia elegans Mast in 2008 9. The objective of the study was to evaluate the safety and effectiveness of this traditional remedy which was used by many local communities as an alternative antimalarial drug in Uganda. The study revealed that the dose had never limited the toxicity of test extracts but the length of time at which the adverse effect was manifested on Balb c mice treated orally 9. Even though the adverse effects of test extracts manifested within a short period of time when the higher doses were administered orally, the adverse effects of test extracts also remained after a long period of time when the lower doses administered in the same route. Therefore, it was unable to decide the minimum lethal dose and the maximum effective dose of test extracts. The result of this research had revealed that there is a credibility gap in the method of data processing in experimental pharmacology that needs to be addressed. In 2018, further experimental studies have been conducted on Balb c mice using one independent and two dependent research variables to address the underlying credibility gap in experimental pharmacology. Correction has been made in this mini review to add the second independent research variable (the body weight of a study animal) which are listed respectively as follows (Table 1) 3.
Administered dose (d),
The body weight of a study subject (w),
Elapsed time (t) for adverse effect manifestation and
The changes in the immunoglobulins immune response after dosing (ΔIg).
The objective of the latest studies was to develop new computational method for systemic biology in order to be able to define the quantitative and qualitative response efficiency of treated study animals against the side effect of administered test compounds. An integrated biology based approach was employed in different studies to analyse dose – biological response relationship 3. First, the acute toxicity of different test chemicals at different levels of doses were evaluated for a maximum period of 5 days during which the time elapsed for the manifestation of significant adverse effect on treated Balb c mice was determined and recorded in note book. The immune response had also been evaluated using quantitative immunoassay before dosing as a reference test and four hours after dosing for a comparison from which the changes in the concentration of serum immunoglobulins (ΔIg) had been calculated and documented. The biological responses against each doses of test chemicals were finally computed as the toxic reaction rate (r) and toxic severity (s) using the next computational formulas respectively; (r=d/t-ΔIg) mg/sec and (s=r/d⨉100) %/sec where d represents the administered dose which determines the magnitude of a biological response, t is elapsed time for adverse effect manifestation and (ΔIg) is the changes in the immune response after dosing.
Please note that, after in-depth review, correction has been also made on the computational formula for the toxic severity (s) mentioned above which was published in the previous articles. The toxic reaction rate (r) of a dose should be divided by the body weight (w) of treated study animal to calculate the severity of a biological harm being caused by the administered dose which is termed as the toxic severity of a dose. On the other hand, the toxic reaction rate (r) should be divided by the amount of administered dose (d) to compute the bioavailability of harmful molecules (h) of a test compound. It represents the proportion of administered dose that has reached drug receptor and has manifested undesirable biological response (Table 5). The toxic reaction rate (r), toxic severity (s) and bioavailability of harmful molecules (h) of a test compound are directly related to the amount of a dose administered into a study animal. The toxic reaction rate (r) of a test compound is the number of a harmful molecule of a test drug that resists the antagonistic effect of immunoglobulins and manifest undesirable biological responses on treated study animals per unit time. It is directly proportional to the number of a harmful molecule of a test drug that binds with the binding domain of a drug receptor and cause undesirable biological responses on study animals. The toxic severity (s) of a test compound, on the other hand, refers to the magnitude of a biological harm or injury caused by the amount of administered dose. Therefore, the computational formula for toxic severity (s) should be corrected as (s=r/w⨉100)%/sec, whereas the bioavailability of harmful molecules (h) of a test compound should be computed as (h=r/d⨉100) %, where w represents the body weight of a study animal, r is the toxic reaction rate of administered dose and d is the amount of administered dose. The qualitative description of toxic severity of a dose reported in the previously published articles remains the same despite the fact that this minor revision could cause some quantitative changes in the previous reports. The following quantitative changes have been made on the data of toxic severity which are listed in Table 3. The author deeply apologizes for the distractive mistake reported in the previous publications.
Table 5. The bioavailability of harmful molecules (h) of a test chemical four hours after treatment of Balb c mice.| Test chemicals | Doses tested | Bioavailability of harmful molecules (h) in % |
| Dichlorvos | 10 mg/kg | -199.0 |
| 50 mg/kg | -19.8 | |
| 90 mg/kg | X | |
| Chlorpyrifos | 10 mg/kg | -299.0 |
| 50 mg/kg | -39.8 | |
| 90 mg/kg | 11.1 | |
| Cypermethrin | 10 mg/kg | -199.0 |
| 50 mg/kg | 20.0 | |
| 90 mg/kg | 33.3 |
The processed data using the above computational methods helps to make relatively precise decision on the pharmacologic property of a test compound at the earliest possible stage of a preclinical trial which may help to avoid unnecessary wastage of time and resources. It also provides quantitative toxicological data by which the toxic severity of a test compound administered to a study animal could be decided to support regulatory categorization and harmful labelling decisions. It helps to process molecular and cellular based toxicological data which again helps to avoid the possible infiltration of harmful products into the market for consumption.
The Role of a Dose in Pharmacology
The results of different researches have demonstrated that there is scientific misconception regarding the role of a dose in pharmacology 3, 9, 10. It is considered as the fundamental concept of toxicology by which the poison of a test compound believed to be avoided which is far from scientific reality 10. The findings in different studies are clearly shown that the dose could neither change nor eliminate the toxicity of a test compound that has been administered into study animals 3, 9. The dose was instead determined the magnitude of a biological response which was again determined the length of time at which the adverse effect was manifested on treated Balb c mice 3, 9. A biological response termed as the toxic severity of a dose which was greater than zero, has killed treated Balb c mice at different length of time depending on its magnitude. This means that the response efficiency of the body would decide the toxicity of a compound that could be expressed as the toxic severity or toxic reaction rate of a dose. Study Balb c mice treated with different doses of test compounds had no equal opportunity to exist in life but equal fate for death at different lifespans. The death of treated Balb c mice was extremely likely following the decline of the immunoglobulins immune response after dosing as an example. These research findings gave the awareness that life has predetermined response efficiency against the side effects of xenobiotics to sustain the different biological activities of the body for a limited period of time depending on the degree of exposure. The dose beyond a biological response that meets the biological need and efficiency of the body would be, of course, a poison. In other words, life could not continue to exist with the biological activity that does not limited to its biological need. This means that the dose of a test compound doesn’t determine the toxicity but a biological condition that fulfils the biological efficiency of the body which actually determines a lifespan. The findings of these studies have revealed potential data that could pave the way to discover the unknown mechanism of ageing.
References
- 1.Wu Quire, Taboureau Olivier, Audouze Karine. (2020) Development of an adverse drug event network to predict drug toxicity. , Current Research in Toxicology 1, 48-55.
- 2. (2015) Basic and Clinical Pharmacology. Drug Receptors and Pharmacodynamics, Mac Graw-Hill education, a LANGE Medical Book; 13th edition. 20-40.
- 3.Y T Belay. (2019) Study of the principles in the first phase of experimental pharmacology: The basic step with assumption hypothesis. , BMC Pharmacol. Toxicol 20, 2-12.
- 4.Vasiliou V, Pappa A, R D. (2000) Petersen. Role of aldehyde dehydrogenases in endogenous and xenobiotic metabolism. , Chemico-Biol. Interact 129, 1-19.
- 6.Schroeder H, Cavacini L. (2013) Structure and function of the immunoglobulins. , J Allergy Clin Immunol 125(202), 41-52.
- 7. (2015) Lippincott Illustrated Reviews, Pharmacology. Drug-Receptor Interactions and Pharmacodynamics; Wolters Kluwer, 6th edition. 25-37.
- 8.Davidson’s principles and practice of medicine, (2014); Therapeutics and good prescribing, Elsevier Limited, 22nd edition. 17-41.
- 9.Belay Y. (2011) Study of safety and effectiveness of traditional dosage forms of the seed of Aristolochia elegans mast against malaria and laboratory investigation of pharmaco-toxicological properties and chemical constituents of its crude extracts, Ann Trop Med Public Health;. 4, 33-41.
- 10.Yilkal Tariku Belay. (2019) Misconception about the role of a dose in pharmacology, Short review report on the biological and clinical effects. , Adv Bioeng Biomed Sci Res (ABBR); 2(3).
